Way back in 2009 (the ScienceBlogs years) I published a Tetrapod Zoology article titled ‘Tell me something new about basilisks, puh-lease’. Aware that there’s all too little squamate content here at Tetrapod Zoology ver 4, I here present a much augmented and updated version of that article…
Caption: captive Emerald or Plumed basilisk, showing the forked cranial crest and tall, serrated dorsal sail and serrated caudal sail of this species, though more ‘extreme’ individuals of this species exist... The light green ground colour and blue markings are characteristic. Note the massive length of the toes. Image: Tina Whitlock, used with permission.
Thanks to their striking appearance, basilisks are often featured in books and on TV, but people only ever say the same two things about them: (1) that they have striking display structures like those so obvious here, and (2) that they can run (bipedally) across the surface of water. Yeah, I’ve heard all that before. Tell me something new!
Basilisks are a group of large, mostly green iguanians from Central and South America*, the biggest specimens of which reach 90 cm in total length. As a generalisation, they’re animals of mature, densely vegetated forests – preferably with running streams – and prefer high temperatures and humidities. They’re strongly arboreal and adapted to climbing on boughs and branches, sometimes many metres from the ground (to 20 m at least; Mora & Escobar-Anleu 2017). They rely on water as a means of escaping from and retreating from predators; more on that below.
* In 1976, the Brown or Striped basilisk Basiliscus vittatus was documented in Florida, its presence here being almost certainly due to escape or intentional release from the pet trade. The species is now well established at several location in the south of the state.
Caption: like many iguanians, basilisks are good at clinging to, and climbing on, vertical and near-vertical trunks and branches, and typically adopt a pose where the palms and soles face inwards. These images show (left to right) two Common basilisks and a Western or Red-headed basilisk B. galeritus. Images: James J. Sharp, CC BY-SA 4.0 (original here); Pavel Kirillov, CC BY-SA 2.0 (original here); Daniel van der Post, open access (original here).
Exactly how much of their foraging and feeding is done in the arboreal environment versus the ground is still unclear as far as I can tell, and some sources imply that their use of the arboreal environment is more to do with predator avoidance and/or basking than specialisation. However, their food preferences and anatomy (read on) mean that they really should be regarded as specialised climbers. It’s also clear that basilisks do a lot of things on the ground. So… arboreal, terrestrial, which is it? And what about the semi-aquatic behaviour (discussed further below)? I just don’t think we have a term for animals that combine habitat use like this. Or do we?
Basilisks sleep on branches and leaves but rare reports of young animals using large riverside boulders as night-time sleeping locations are on record too (Mora & Escobar-Anleu 2017).
Caption: montage featuring three different Common basilisks. The juxtaposition of longitudinal white stripes and transverse dark ones is obvious in this species, and note also the variation in sail size and shape. Images (clockwise from upper left): The Rambling Man, CC BY-SA 3.0 (original here); Daniel Hincapie, CC BY 4.0 (original here); Anthony Batista, CC BY 4.0 (original here).
No, basilisks are not aquatic. And having mentioned riverside boulders… basilisks are (mostly) associated with a specific kind of tropical forest: that adjacent to bodies of water, including ponds, streams, rivers, sinkholes and even the sea. Individuals are very often found within 1-2 m of the water’s edge (Lattanzio & LaDuke 2012) but they can’t be regarded as limited to water-edge microhabitats given that adult Brown basilisks are very often observed some distance from water. Furthermore, the Emerald or Plumed basilisk B. plumifrons of Mexico and Central America is seemingly less tied to water than the other species and often occurs well away from it.
Caption: Western or Red-headed basilisk photographed in a stream. Basilisks of most (but not all) populations forage close to, or in, streams and other water bodies, meaning that it might be right to describe them as semi-aquatic. But it might not. Image: desertnaturalist, CC BY 4.0 (original here).
It absolutely isn’t correct to describe basilisks as ‘aquatic’ (seeing as they don’t do anything like live in water or on its surface), but they have been reported to forage in streams and other bodies of water for aquatic insect larvae and crayfish, a consequence being that some authors have described them as ‘semi-aquatic’. They’re also capable divers and the Brown basilisk has been reported to disappear underwater as a predator avoidance tactic. The long hindlimbs, overall proportions and long, heavy tail mean that they can run bipedally if speed and space allows, and here’s where we come to the water-running behaviour.
On being startled or alarmed, a basilisk will drop into the water and quickly skitter across the surface, its body inclined upwards and forelimbs mostly clear of the water. This water-running behaviour works best among small, lightweight juveniles who are able to stay more elevated from the water surface than are large, heavy adults (who can weigh up to 600 g). The key to water-running is the presence of rectangular fringes on the toes that trap air and prevent the foot from being pushed too far into the water, a swift, circular recovery stroke that allows the limb to be extracted and returned to starting position with minimal disruption of the water, and a stable body position that keeps the centre of mass between the supporting limbs (Glasheen & McMahon 1991, 1992, Hsieh & Lauder 2004).
Caption: the famous water dashes performed by basilisks happen so quickly, and occur over such short distances, that it’s hard to capture them adequately without high-speed photography. Image: Ted, CC BY-NC 2.0 (original here).
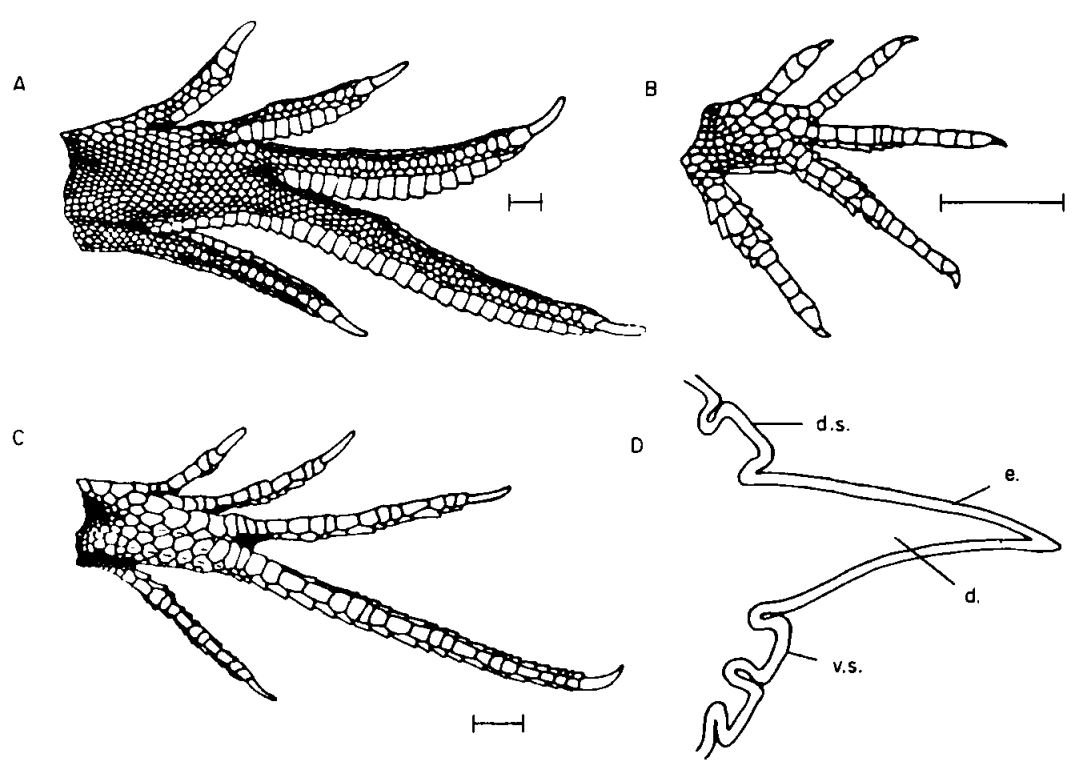
How did such a remarkable set of features evolve, given that intermediate stages would disallow water-running behaviour and thus make basilisks vulnerable to predation from some of the animals they most want to avoid (like fishes and large birds)? A good argument has been made that basilisk toe fringes are an excellent example of exaptation, this being the evolutionary phenomenon whereby features that evolve in the context of one behaviour later prove advantageous for another, and hence get co-opted. Rectangular toe fringes have evolved at least five times in arboreal lizards specialised for fast movement on leaves, including in agamids, tropidurines, lacertids and teiids, and they’re present in corytophanids other than basilisks too (Vitt in Pianka & Vitt 2003). Toe fringes are widespread in lizards, though more often involve triangular or conical scales (Luke 1986), not the unusual rectangular scales relevant here.
Caption: rectangular toe fringes (actually formed of projecting scales that are in close contact) as depicted in several non-basilisk lizards by Luke (1986). (A) the agamid Hydrosaurus, (B) the African lacertid Holaspis, (C) the teiid Kentropyx, and (D) cross-section of a Hydrosaurus fringe, showing how one of the scales forming the fringe projects way out horizontally relative to the rest of the toe. Scale bars = 10 mm. Image: Luke (1986).
The conclusion has to be that the fringes are there due to arboreal specialisation, but later provided an advantage in a specific escape strategy involving water. Basilisks now rely on this strategy and use it routinely; other fringe-toed taxa can do the water-running thing as well but are not as good at it and only use it opportunistically (Vitt in Pianka & Vitt 2003).
Caption: close view of the face of a Common basilisk. Not sure why this individual has a white crest. Image: Brian Gratwicke, CC BY 2.0 (original here).
Taxonomy, phylogeny, diversity. Four basilisk species are presently recognised: the Common basilisk B. basiliscus, the Western or Red-headed basilisk B. galeritus, the Emerald or Plumed basilisk B. plumifrons and the Brown or Striped basilisk B. vittatus. Subspecies have been recognised for some of them and it’s suspected that there are additional ‘species-worthy’ populations here. I’m thinking in particular of a Colombian population currently included in the Red-headed basilisk. It looks unique due to its especially large, rounded cranial crest.
Caption: the Western or Red-headed basilisk, probably the least familiar and most unusual of basilisks (at least, to those of us outside the American tropics). Note the serrated dorsal crest and very long, slender limbs. Images (left to right): Sandy Martinez, CC BY 4.0 (original here); desertnaturalist, CC BY 4.0 (original here).
A phylogenetic study that incorporated molecular, anatomical and sperm morphology data found the Red-headed basilisk to be outside a clade that contained all the others, and the Emerald and Common basilisks to be sister-taxa (Vieira et al. 2005). The latter two are the most ‘extreme’ of basilisks in morphology (the Red-headed and Brown lack the massive dorsal and tail frills of the Emerald and Common basilisks, and are smaller than them as well), so this makes sense. However, a more recent study (Taylor et al. 2017) found Brown and Common basilisks to be sister-taxa, in which case frills evolved twice, or were lost in the lineage leading to the Brown basilisk.
Caption: male Common and Emerald basilisks compared, both photographed in Costa Rica. Note the different attitude of the dorsal sail in these two individuals. Because the sails are supported by neural spines, they aren’t under muscular control. Images: Derkarts, CC BY-SA 3.0 (original here); Connor Long, CC BY-SA 4.0 (original here).
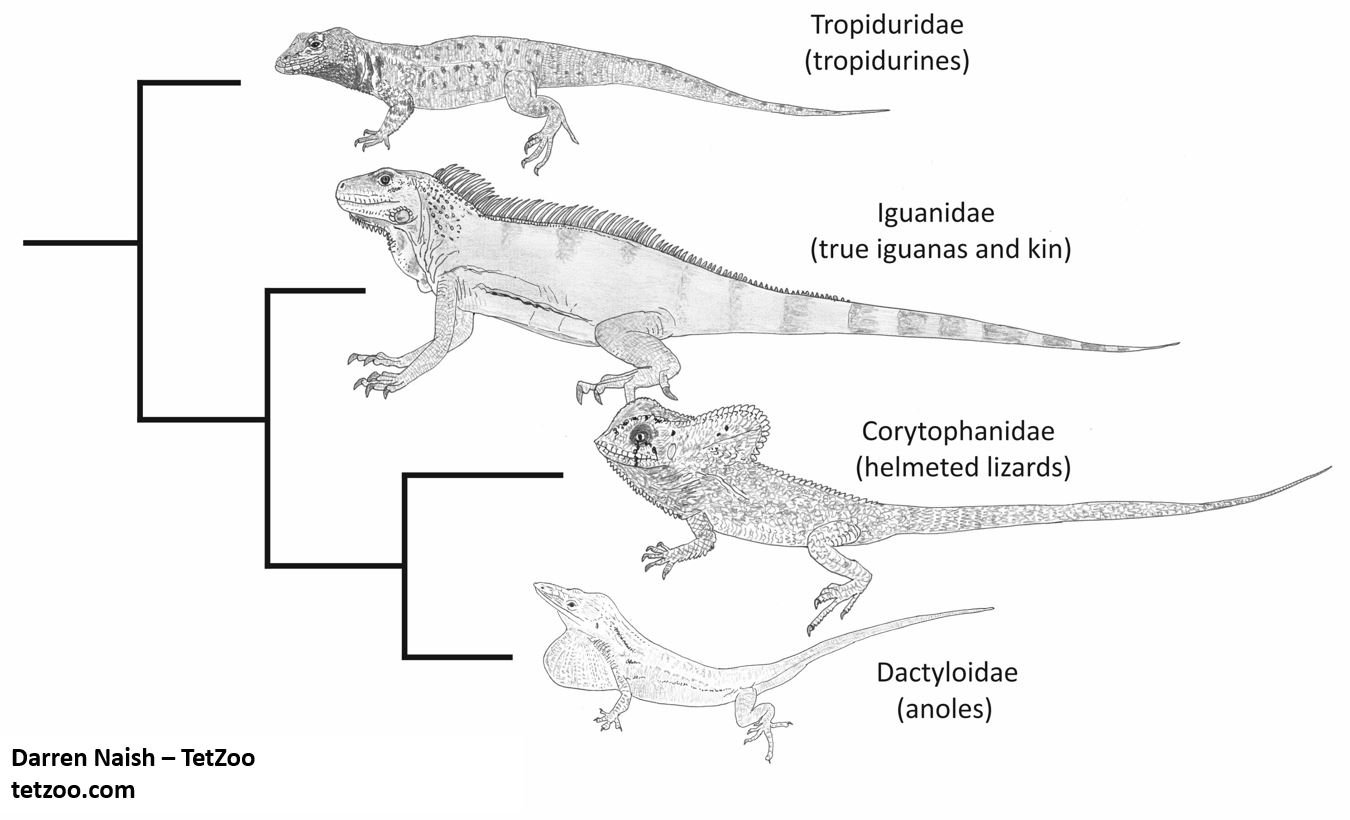
Caption: highly simplified hypothesis of phylogeny relating to corytophanids and their close relatives. For much of the late 20th century, all of these groups were lumped together within ‘Iguanidae’, though the trend today is to recognise the main constituent clades as ‘family level’ groups. This cladogram and its illustrations are from my in-prep textbook, though that green iguana has broken proportions and needs replacing. Image: Darren Naish.
In the larger picture, basilisks are part of the iguanian group Corytophanidae, a clade that includes three extant genera (casquehead iguanas Laemanctus and helmeted iguanas Corytophanes are the other two) (Lang 1989, Frost et al. 2001, Vieira et al. 2005) (the same group was termed Basiliscinae in the older literature: see Lang 1989). Experts have disagreed on whether corytophanids originated in South America (and ultimately in Gondwana) and moved north, or whether they came from the Mexican Plateau or thereabouts and moved south. By combining a phylogenetic hypothesis with distribution data, Vieira et al. (2005) argued that corytophanid evolution was a Central American event and that basilisks invaded South America at least twice, once in or after the middle Pliocene (giving rise to the endemic South American Red-headed basilisk) and at some point more recently (giving rise to South American populations of the Common basilisk).
A very different hypothesis was put forward by Taylor et al. (2017) who found all species-level splits within Basiliscus to have occurred in the Early Miocene or the preceding Oligocene (between about 35 and 20 million years ago).
Caption: time-calibrated corytophanid iguanian cladogram from Taylor et al. (2017). The North American fossil taxon Babibasiliscus is nested within the crown (though the European Geiseltaliellus is not). Within basilisks, Brown and Common basilisks are sister-taxa. Image: Taylor et al. (2017), CC.
But then… fossils! Fossils further complicate things. 2015 saw the publication of the fossil corytophanid Babibasiliscus alxi from the Lower Eocene Bridger Formation of Wyoming. Babibasiliscus was argued by Conrad (2015), its describer, to be a crown-corytophanid, closer to Laemanctus than are Corytophanes and Basiliscus. Another Eocene lizard – Geiseltaliellus from Germany, France and Belgium (named back in 1944) – was found to be part of the crown too, and closer to Corytophanes and Laemanctus than Basiliscus. Other studies have, however, found Geiseltaliellus to be outside crown-Corytophanidae (Taylor et al. 2017).
Caption: time-calibrated corytophanid phylogeny from Conrad (2015), showing skulls of the relevant taxa. Image: Conrad (2015).
These fossils – found so far from the Central American region otherwise considered integral to corytophanid history – are important, since they could mean that Central America simply represents that region where corytophanids ‘ended up’. It isn’t, perhaps, their ancestral ‘home’ or ‘site of origin’. Two main ways of explaining corytophanid distribution seem to exist. One is to stick with the conventional view that they originated and diversified in Central America, but to posit that one or two lineages moved away from that region and colonised the north and – to the far east – Europe (via the famous/infamous De Greer land bridge). And another is to think that corytophanids were ancestrally present across much of Europe and North America. If the latter is so, the living taxa are relicts or southerly occurring members of a previously more ‘northerly’ clade.
Worth mentioning here as an aside is that a group of extinct iguanians from the Eocene and Oligocene of Europe and Oligocene of North America – the messelosaurines – are apparently part of Corytophanidae too (Rossmann 1999a, b, 2005). Rossmann (1999a) proposed that corytophanids originated in Europe and ended up in South America following dispersal to North America.
Caption: female Common basilisk photographed on the banks of the Chagres River, Panama. The prominent white striping and great length and slender nature of the tail are really obvious here. Image: Charles J. Sharp, CC BY-SA 4.0 (original here).
Anatomy. Basilisks are highly distinctive anatomically. The cranial crest is unique, as are the tall, serrated dorsal and caudal fins. The cranial crest is supported internally by a sheet-like outgrowth of the parietal and is largest in adult males, though only Emerald and Brown basilisks are truly sexually dimorphic when size-related differences are accounted for (Taylor et al. 2017).
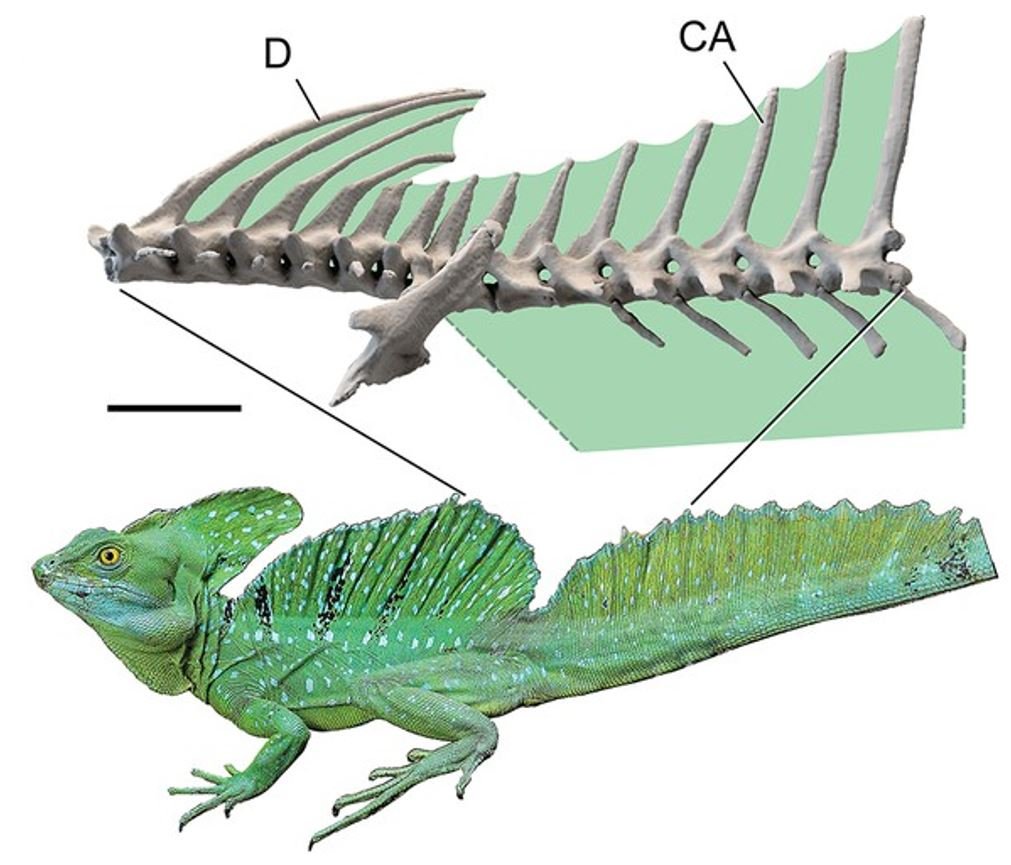
The dorsal and caudal fins are supported by long, slender neural spines that extend all the way to the serrated fin margins, with those of the dorsal region sometimes having triangular bases that are distinct from the spine-like, more dorsal parts (Lang 1989, Sereno et al. 2022). The superficial similarity that these spines have with the tall neural spines of the Cretaceous dinosaur Spinosaurus has not been missed and the fact that basilisk fins are irrelevant to aquatic behaviour has been used by those arguing that Spinosaurus was not aquatic (Sereno et al. 2022).
Caption: mounted and posed basilisk skeleton – I presume of Emerald or Plumed basilisk – made by The Skeleton Factory. Image: (c) The Skeletal Factory.
An interesting aside about their flamboyant appearance is that captive basilisks tend not to develop the crests and sails to a prominent degree. This has been blamed on lighting, diet and stress based on captivity, transportation and/or proximity to people. I don’t know if this issue has been solved, but it might have been given that there are at least some captive males with fairly impressive crests and fins.
Caption: basilisk neural spines involved in support of the dorsal and caudal spines, from Sereno et al. (2022). The life photo shows an Emerald basilisk; Sereno et al. (2022) CT-scanned a member of this species, and I think the diagram also represents that species. D = dorsal neural spines. CA = caudal neural spines. Image: Sereno et al. (2022).
Basilisks are capable of caudal autotomy, which is surprising in view of how important the tail appears to be in locomotion and display.
Behaviour and ecology. As is typical for so many tropical lizards, relatively little is known about basilisk ecology and diet. They are omnivorous and eat certain leaves, flowers and fruit in addition to arthropods. Insects form the bulk of their diet, these variously including grasshoppers, beetles, ants, wasps and caterpillars (Hirth 1963, Fleet & Fitch 1974). Harold Hirth’s 1959-1961 study of basilisks living on the Caribbean coast of Costa Rica is really interesting because he found ants to be the commonest food items recovered from basilisk stomachs, in some cases (6 males and 4 females) filling the stomachs entirely. Basilisks don’t look like ant-eaters and you might not predict that ants are that important to them, yet here we are. Additional interesting arthropod prey include freshwater crayfish, beach-dwelling amphipods, and barnacles that were being opened and dismembered by ghost crabs (Hirth 1963).
Caption: close-up profile view of a captive Common basilisk photographed at Buffalo Zoo. As ever for lizards, the complexity of scale arrangement is bewildering. The massive size, bright yellow colour and bulging nature of the eye is also interesting. Image: Becker1999, CC BY 2.0 (original here).
Basilisks are large and powerful enough to sometimes consume vertebrates, and frogs, snakes, other lizards, and even birds and rodents are eaten on occasion. A Common basilisk was observed swallowing “a hummingbird nest complete with young hummingbirds” (Glander 1979, p. 235). Because basilisks sometimes forage in streams, pools and on beaches, they also prey on aquatic animals and small fish have been reported as prey in both juvenile Common and Banded basilisks (Echelle et al. 1972, Fleet & Fitch 1974). There are photos online showing basilisks consuming juvenile slider turtles.
Caption: Emerald basilisk apparently consuming a juvenile green iguana. I know nothing of the circumstances of this photo (which has been widely shared online) and assume that it’s genuine. Image: (c) Randy Alvarado/Reptilehunter.
Cannibalism is a recorded part of their behaviour too and might even be important given that juveniles and adults sometimes inhabit the same parts of a habitat and sometimes occur in high densities (Lattanzio & LaDuke 2012). Another interesting aside is that sand has repeatedly been discovered within the stomachs of Costa Rican Common basilisks, and in fact occurs so consistently that it’s been regarded as a digestive aid by some authors (Fleet & Fitch 1974). Elsewhere in tetrapods, the ingestion of sand has been linked to insectivory, the idea being that it helps break up the chitinous bodies of insects like ants. Might that be what’s going on here? Or is the ingestion of sand an accidental and inevitable consequence of capturing arthropods on beach sand?
Basilisks of course live alongside diverse other forest-dwelling vertebrates, including other lizards as well as birds, primates and more, and may compete with them for resources. We don’t have much direct data on how the relevant interactions play out but basilisks and birds like thrushes don’t like one another and use chasing and surprise tactics to deter the other from feeding sites (Young 2014). Among non-aggressive observations, Glander (1979) reported Common basilisks feeding on fruit (specifically, on pedicels attached to the fruit) dropped by foraging howler monkeys and Hirth (1963) described basilisks sharing foraging sites (beaches) and sleeping locations with ameivas and green iguanas.
Basilisks themselves are prey for a list of animals, including snakes (like cornsnakes, racers and indigo snakes), herons, black hawks and ghost crabs. Largemouth bass and snooks have been seen to predate on basilisks that were running across water (Flaherty & Friers 2014).
Caption: I mentioned at the top of the article that some Emerald or Plumed basilisks are even more ‘extreme’ than some of the individuals we’re already seen. And here’s an incredibly flamboyant individual, with a super-sized cranial crest and dorsal and caudal sails of Spinosaurus-like proportions. Image: Bernard Dupont, CC BY-SA 2.0 (original here).
Courtship and reproduction. As elaborately ornamented species with (sometimes) obvious sexual dimorphism, we would predict that basilisks are competitive animals where males display during the breeding season and vie for female attention, perhaps in arboreal display areas of the sort known for green iguanas. Males are indeed highly competitive and reported to fight fiercely at times (Fitch 1981) but I’m not aware of observations pointing to lek-type displaying or anything like that. Head bobs are used to signal sexual interest. A size-based dominance hierarchy is established in an area, and smaller males are prevented from breeding by the aggressive actions of larger ones.
Caption: two male Common basilisks in an aggressive encounter, photographed in Manuel Antonio National Park, Costa Rica. Both lizards are elaborate and note the variation in striping. Also relevant is that both animals are on the ground, not in trees. Image: Michelle Reback, CC BY-SA 3.0 (original here).
Basilisks are oviparous and females produce clutches of between 5 and 18 eggs. At least one species (the Common basilisk, specifically a Colombian population of the subspecies B. basiliscus barbouri) appears to be capable of parthenogenesis (Böhme 1975, Lang 1989). Several females, kept captive for a prolonged period and without access to males, successfully reproduced and all offspring were female. As expected, basilisk eggs are buried in moist soil on the forest floor: the nest isn’t just a pit, but a tunnel more than 30 cm long. There are anecdotes in the pet trade (go here) describing female basilisks observing their nests after backfilling it to ensure that the nest area matches the look of the surrounding forest floor sediment. That’s a sophisticated piece of behaviour that might seem beyond the cognitive abilities of most lizards, but on the other hand I wouldn’t say that it’s unbelievable.
Hatchlings emerge after about two months of incubation and live together for some time after hatching, even sleeping piled up on top of one another. Similar behaviour is known for other iguanians (see the Tetrapod Zoology article Amazing Social Life of the Green Iguana).
Caption: all too little study has been done on the behaviour of iguanian hatchlings, but it might be that the sociality and group-living described for green iguanas is widespread within the group. For more information on the images here, see Amazing Social Life of the Green Iguana.
And that just about wraps up everything I wanted to say. I’m very fond of these large, attractive lizards and hope to see them in the wild some day. As should be clear, there’s a lot to say about basilisks beyond the water-running behaviour, and even that turns out to be more complex and nuanced than usually explained. As ever, it’s also obvious that a great many questions remain unanswered or (at best) only superficially or anecdotally answered about these lizards, including certain issues of ecology, habitat use and diet.
Caption: I adore elaborately ornamented lizards, but good scale figures or toys of them are scarcely available. I own a few basilisk toys and they’re all pretty terrible. This one (which is quite large) looks like it was made by the same people who make those ‘roaring’ dinosaurs with projecting point teeth. Image: Darren Naish.
Iguanian lizards have been covered a few times before at Tetrapod Zoology. For previous articles, see…
Harduns and toad-heads; a tale of arenicoly and over-looked convergence, December 2006
Ermentrude the liolaemine, February 2008
‘Cryptic intermediates’ and the evolution of chameleons, June 2008
Amazing social life of the Green iguana, September 2012
The Squamozoic actually happened (kind of): giant herbivorous lizards in the Paleogene, June 2013
Leiosaurus: big heads, bold patterns, October 2013
Grassland earless dragons, January 2014
Australia, land of dragons (by which I mean: agamids) (part I), January 2014
Australia, land of dragons (part II), February 2014
What's With All These New Chameleon Names?, Part 1, February 2016
By the Horns of Trioceros, the Casque of Calumma, the Brood of Bradypodion--Chameleons, Part 2, February 2016
Palleon, Archaius, Kinyongia, Nadzikambia--The Last Chameleons, Part 3, March 2016
The Tropidurine Treerunners, December 2017
The Tet Zoo Guide to Mastigures, August 2018
Refs - -
Böhme, W. 1975. Indizien für natürliche Parthenogenese beim Helmbasilisken, Basiliscus basiliscus (LINNAEUS 1758) (Sauria: Iguanidae). Salamandra 11, 77-83.
Echelle, A. A., Echelle, A. & Fitch, H. S. 1972. Observations of fish-eating and maintenance behavior in two species of Basiliscus. Copeia 2, 387-389.
Flaherty, J. P. & Friers, J. 2014. Predation on the Brown Basilisk (Basiliscus vittatus) in South Florida. Southeastern Naturalist 13, 57-58.
Fleet, R. R. & Fitch, H. S. 1974. Food habits of Basiliscus basiliscus in Costa Rica. Journal of Herpetology 8, 260-262.
Frost, D. R., Etheridge, R., Janies, D. & Titus, T. A. 2001. Total evidence, sequence alignment, evolution of polychrotid lizards, and a reclassification of the Iguania (Squamata: Iguania). American Museum Novitates 3343, 1-38.
Glander, K. E. 1979. Feeding associations between howling monkeys and basilisk lizards. Biotropica 3, 235-236.
Glasheen, J. W. & McMahon, T. A. 1992. A hydrodynamic model of locomotion in the basilisk lizard. Nature 380, 340-342.
Glasheen, J. W. & McMahon, T. A. 1992. An analysis of aquatic bipedalism in basilisk lizards. American Zoologist 32, 144.
Hirth, H. F. 1963. The ecology of two lizards on a tropical beach. Ecological Monographs 33, 83-112.
Hsieh, S. T. & Lauder, G. V. 2004. Running on water: three-dimensional force generation by basilisk lizards. Biophysics and Computational Biology 101, 16784-16788.
Lang, M. 1989. Phylogenetic and biogeographic patterns of basiliscine iguanians (Reptilia: Squamata: “Iguanidae”). Bonner Zoologische Monographien 28, 1-172.
Lattanzio, M. S. & LaDuke, T. C. 2012. Habitat use and activity budgets of Emerald basilisks (Basiliscus plumifrons) in northeast Costa Rica. Copeia 2012, 465-471.
Luke, C. 1986. Convergent evolution of lizard toe fringes. Biological Journal of the Linnean Society 27, 1-16.
Mora, J. M. & Escobar-Anleu, B. I. 2017. River rocks as sleeping perches for Norops oxylophus and Basiliscus plumifrons in the Cordillera de Talamanca, Costa Rica. Mesoamerican Herpetology 4, 418-422.
Pianka, E. R. & Vitt, L. J. 2003. Lizards: Windows to the Evolution of Diversity. University of California Press, Berkeley.
Rossmann, T. 1999a. Messelosaurine lacertilians (Squamata: Iguanoides) from the Palaeogene of France and North America. Neues Jahrbuch fur Geologie und Paläontologie, Monatshefte 1999, 577-592.
Rossmann, T. 1999b. "Crotaphytus" oligocenicus (Holman, 1972), (Squamata: Iguanoidea) from the Oligocene of Saskatchewan, reinterpretation and some paleobiogeographical implications. Neues Jahrbuch fur Geologie und Paläontologie, Monatshefte 1999, 186-192.
Rossmann, T. 2005. Nachtrag zur Osteologie und Palaobiologie von Geiseltaliellus longicaudus Kuhn (Lacertilia, Iguanoidea) aus dem Mittleren Eozän (MP 11) der Grube Messel, nahe Darmstadt. Courier Forschungsinstitut Senckenberg 255, 225-236.
Young, A. M. 2014. Basiliscus plumifrons (double-crested basilisk lizard) antagonistic behavior. Herpetological Review 45, 694-695.