Among the most poorly known of all squamate groups are the dibamids…
Caption: a dibamid exemplar. Note the smooth-scaled, shiny overall look, the essentially eyeless head, and the pale patches on the snout and scattered across the body. This specimen, from the Philippines, was identified as Dibamus cf leucurus, so hadn’t been pinned down to species when published. Image: Brown et al. (2016), CC BY 4.0 (original here).
… a group so obscure that they don’t really have a common or vernacular name (though read on). Little information on the group is available, and in this article I aim to cover essentially everything about them that I consider worth saying…
What are dibamids? They’re near-limbless, fossorial, snake-shaped, near-blind, insectivorous squamates mostly occurring in southern and south-eastern Asia (including the Wallacean region as far east as New Guinea). A single species – the Mexican blind lizard Anelytropsis papillosus – occurs in eastern Mexico. Excluding Anelytropsis, all the other species are included within the genus Dibamus*. Members of both genera have been known to science for some time: Dibamus was named by French zoologists André Marie Constant Duméril and Gabriel Bibron in 1839 for D. novaeguineae (originally described from New Guinea, in case you’re guessing) while Anelytropsis was first published by Edward Cope in 1885.
Within recent decades, the number of dibamid species has increased quite markedly. Books I recall from childhood (e.g., Whitfield 1983) said that there were just three species, a 1985 review increased this to nine (Greer 1985), and the steady and gradual description of new species since then has put us at 26 species as of May 2024. It’s not unlikely that the count will double within the next few decades.
Caption: the holotype specimen of Dibamus dalaiensis, discovered in the southwestern Cardamom Mountains, Cambodia, and described by Neang et al. (2011). It confirmed the presence of this group in Cambodia. Those patches that you might assume are areas where the skin is sloughing are actually pale grey areas, and a normal feature of the pigmentation. Note also the slight iridescent sheen on the dorsal reflective areas. Image: Neang et al. (2011).
* A notorious taxonomic vandal, whose work cannot be taken seriously and whose taxonomic proposals should be ignored, has proposed several genus-level names for clades within Dibamus. For reasons discussed below, it’s almost inevitable that Dibamus will indeed undergo taxonomic revision at some point. When that happens, however, we won’t be using the names proposed by the taxonomic vandal alluded to here (see Kaiser et al. 2013, Rhodin et al. 2015).
All dibamid species are less than 25cm long, and a curious feature is that the tail is proportionally short, usually being less than one-third SVL (snout to vent length) and less than 10% of SVL in some Dibamus species (Greer 1985). Snakes are short-tailed relative to SVL, so this is a snake-like character.
Caption: I’ve said several times in older articles that my very first introduction to a good number of amphibian and reptile species came via Philip Whitfield’s Reptiles and Amphibians: An Authoritative and Illustrated Guide of 1983, and more specifically to the astonishing art by Alan Male. In cases, it was obvious that Male only had access to vague or poor reference photos or other images, and I think we can say that this was the case with his dibamid illustration, shown in the book on the same plate as anguids and anniellids. When the text was written, only three dibamid species were recognized. Image: Alan Male, from Whitfield (1983).
As ever with obscure reptiles, good luck if you want to find a comprehensive source that says much about these animals. Most books that cover squamate diversity mention dibamids in passing and don’t dwell on the things that make them interesting. The standard and best reference on them remains Allen Greer’s Journal of Herpetology paper from 1985, and I relied on it heavily here. It is fair to say, however, that dibamid skull anatomy is sufficiently unusual that many people interested in squamates have had reason to investigate it and ponder on what it might mean for squamate biology and evolution more broadly (Rieppel 1984, Greer 1985, Hallermann 1998).
Having said that dibamids don’t have a common name, both ‘blind lizard’ and ‘blind skink’ have been used. But both are less than ideal: ‘blind lizard’ is similar to ‘blind worm lizard’ used for some amphisbaenians (which are a very separate group, not closely related), and ‘blind skink’ is also not good since dibamids are not skinks nor close to them either.
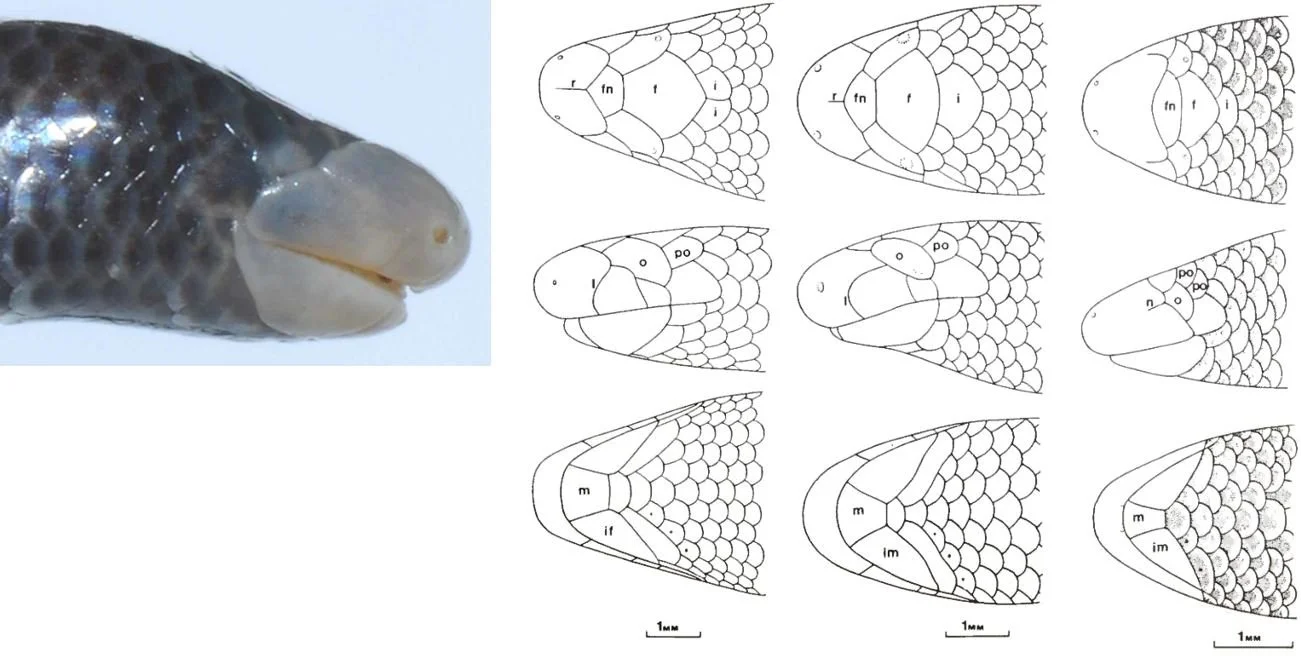
Caption: at left, the head of D. dalaiensis from Cambodia in right lateral view, showing the prominence of the rostral and mental pads. At right, a montage showing the heads of (left to right) the D. greeri holotype, the D. greeri paratype, and the D. smithi holotype. The heads are shown in dorsal (upper row), left lateral (middle row) and ventral (lower row) views. The scale bar is 1 mm. Various of the scales have been labelled, including ocular (o), postocular (po), frontal (f) and mental (m) (there’s an error: some lower jaw scales labelled ‘im’ should be labelled ‘if’ for infralabial). All the main scales on the squamate head have names, and its typical to refer to their form and placement in descriptive discussions. Images: Neang et al. (2011), Darevsky (1992).
Dibamid anatomy, a primer. What are dibamids like in anatomical terms? Live dibamids are blunt-snouted, blunt-tailed, shiny-scaled squamates that range from purplish to dark brown, the underside being paler. The tongue is not bifid, but broad and fleshy. Pale patches are present across the body in some species. A peculiarity of some species – like D. greeri from Vietnam (named after the aforementioned Allen Greer) – is the presence of bright blue rings on the body. Darevsky (1992) noted that these can’t have any role in intraspecific signalling (given that dibamids are nearly blind, and subterranean too), so might represent mimicry of sympatric megascolecid earthworms, some of which possess unusually coloured body rings that are apparently toxic. Birds including spurfowl, peafowl and junglefowl might, Darevsky (1992) speculated, therefore avoid predating on dibamids… or, on some dibamids, anyway, and Darevsky (1992, p. 8) did bring attention to the un-tested and speculative nature of this hypothesis.
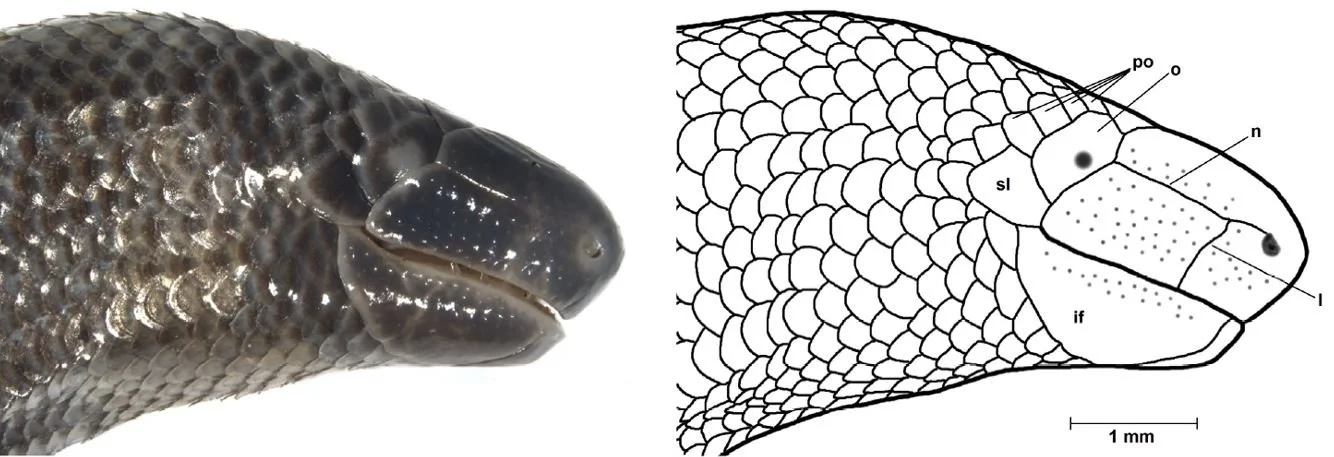
Caption: a beautifully detailed photo and diagram of Dibamus manadotuaensis, a species from Manado Tua, a small island off the northern coast of Sulawesi, Indonesia named in 2019. Note the tiny size and the small sensory papillae scattered across the surfaces of the large face plates. Extra points if you think this looks like the 2014 Gareth Edwards Godzilla, since it totally does. Image: Koppetsch et al. (2019).
Thick-skinned, milky-coloured plates occur across the anterior halves of the dibamid snout and lower jaw. Greer (1985) termed these the rostral and mental pad, respectively. They aren’t homogenous across their surfaces since they’re broken up by sutures (the distribution of which varies from one species to the next), and a “large number of evenly distributed sensory papillae” (Greer 1985, p. 121) also occur across their surfaces. The implication is that these structures have some kind of sensory role, but one that’s uninvestigated so far as I can tell. A pale area corresponding to the eye is present in some species but not all.
The dibamid skull is rigid (as is typical for burrowing squamates), has closed fenestrae (again, as typical for burrowers), and is tiny at just 5-7 mm in length. Numerous details are unusual relative to the condition present in other squamates. The bony palate is one of the most ‘complete’ within Squamata, extending as far posteriorly as the anterior edge of the braincase, albeit with a midline furrow at its rear margin. This is occupied by spongy, possible glandular, tissue in life (Greer 1985). Within the snout chamber, scroll-like structures and bony laminae enclose the nasal passages (Greer 1985, Hallermann 1998). Similar configurations are seen in the fossorial acontine skinks and the unusual feylinines (Greer 1985) – there are some similar laminae in uropeltid snakes as well – so presumably this is something to do with breathing while burrowing through sediment.
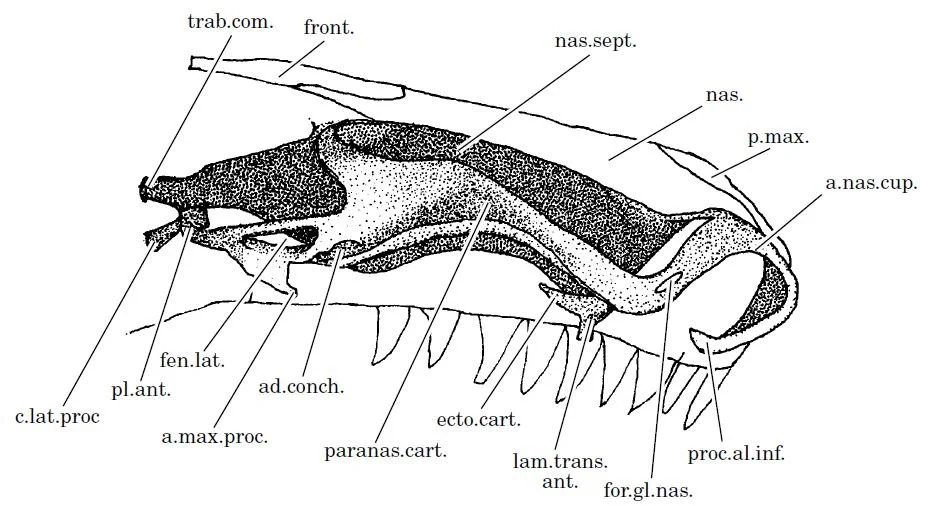
Caption: it’s not often that you get to see the cartilaginous nasal capsule within the snout of a squamate. This diagram – from Hallermann (1998) – was based on reassembled serial sections. The nasal capsule – the sinuous structure closest to us in the diagram – is paired, and each one is separated along the midline by a septum (nas. sept.). Each capsule has a complex relationship at the snout’s tip with the septum, the capsule forming a curled anterior nasal cupola (a. nas. cup.). Hallermann (1998, p. 389) suggested that this complex anterior anatomy might be “a consolidation in adaptation to burrowing habits”.
The lower jaw is simplified (being formed of just three bones, the most posterior of which looks to be a compound amalgamation of the ancestrally separate five), and has a low tooth count, there being 8-10 teeth (on one side) depending on the species. The tooth count in the upper jaw is harder to discuss. Why? Because the conjoined premaxillae (the bones at the front of the upper jaw) sometimes have an odd number of teeth (7) due to the presence of a midline tooth. This feature is not unique within squamates since it also occurs within some geckos – remember this point!
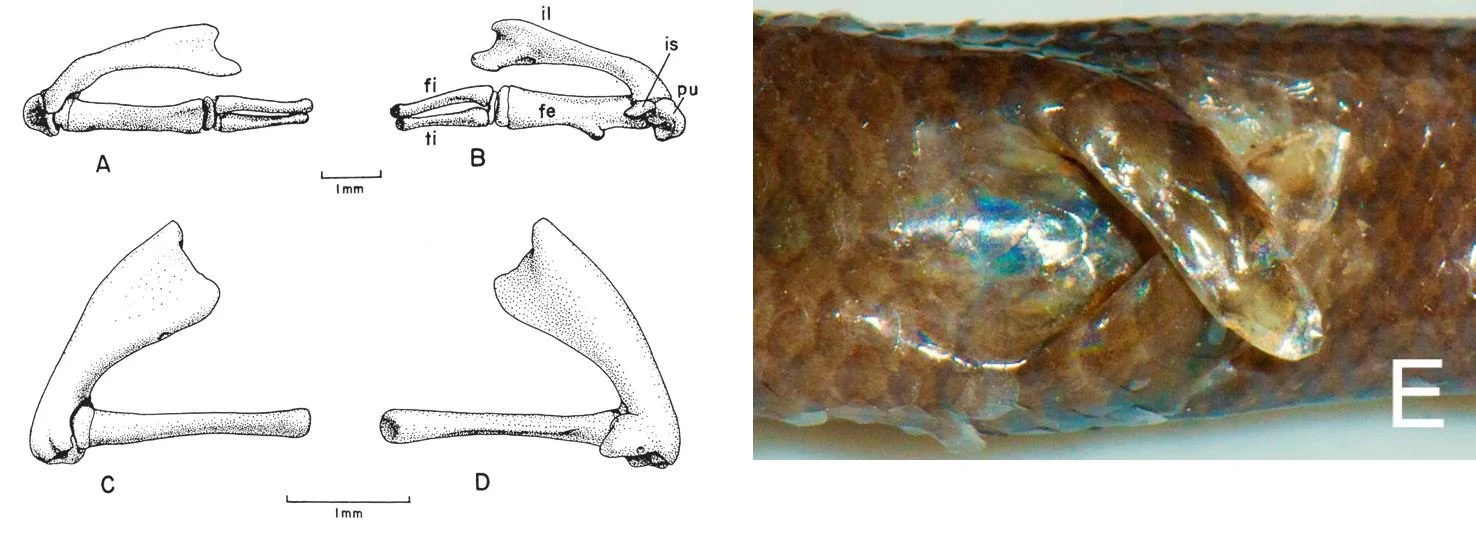
Caption: dibamids can’t be described as limbless. At left, the hindlimb and pelvis of male Dibamus specimens, as figured by Greer (1985). A and B show bones from a male D. taylori (with fibula and tibia); C and D show bones from a female D. novaeguineae. The scale bars are 1 mm. The pelvic girdle is mostly formed of a large ilium (il), with a tiny pubis (pu) and ischium (is) persisting as small ossicles visible in B. At right, the flipper-like hindlimbs of a male D. floweri from peninsular Malaysia, a species described in 2017. I don’t think it’s typical for the hindlimbs to be crossed like this. Images: Greer (1985), Quah et al. (2017).
Another anatomical area of interest concerns limbs, or their absence. Forelimbs are wholly lacking and only a relictual, splint-like pectoral girdle remains. On the issue of hindlimb presence or absence, dibamids are ambiguous, one reason being that they’re sexually dimorphic on this point: females lack limbs entirely, but males have small, flap-like hindlimbs that they use to grip females while mating. On bones, males have a femur, tibia and fibula (plus some attendant cartilage caps) while females just have a femur. Anelytropsis is variable with respect to all this, some males having a tibia and fibula and others being like females in lacking them (Greer 1985). One final thing on skeletal anatomy: it’s been stated that osteoderms are present in some dibamids but this was regarded as a mistake by Greer (1985).
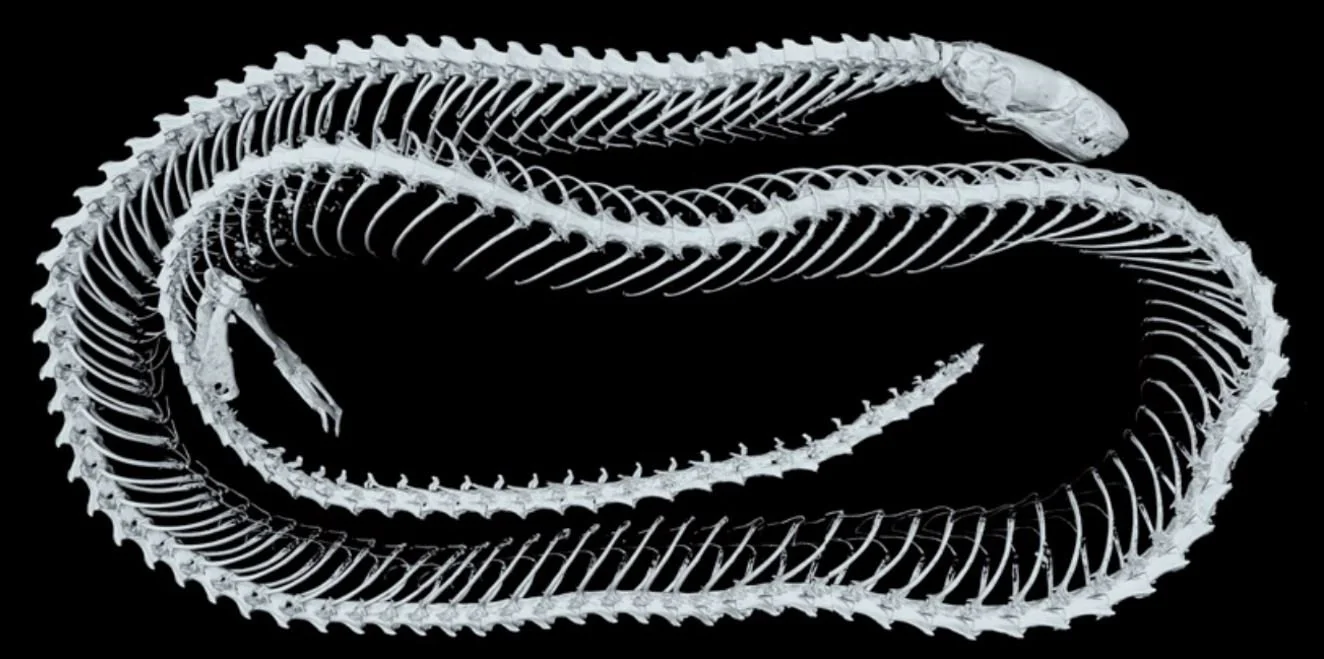
Caption: the complete skeleton of the recently named Vietnamese dibamid Dibamus deimontis, specifically the male paratype. It’s 15.3 cm long in total. Note the relatively well developed hindlimb (though lacking a foot) and pelvis, and the relatively short length of the tail. Image: Kliukin et al. (2024).
Reduced eyes, lone lungs and erectile scales. Dibamid eyes are reduced (no sclerotic ring, no lens, no iris, no vitreous body), covered by scales, and presumably only have a role in light sensing. Both external ears and a middle ear cavity are absent.
On organs, dibamids are like some amphisbaenians in only having a single lung: on the left side, it’s anterior to the heart, but on the right side it’s posterior to the heart. This is different from the condition in snakes, since in them both lungs are generally present and it’s the right lung that’s larger and more important than the left. Alveoli are apparently absent in the dibamid lung (Greer 1985), as is the case in snakes. Another snake-like feature is that the heart is situated far back in the body (relative to what we mammals are familiar with).
An especially notable dibamid feature concerns the ability of at least some species to elevate their body scales such that they project almost perpendicularly from the body. This gives them a rugose or wrinkled appearance: one suggestion is that this is another convergence with megascolecid earthworms that inhabit the same environment, since some of those are bristle-covered (and presumably toxic) (Diaz et al. 2004, Kliukin et al. 2023). Prior to reading about this, I wasn’t aware that the scales of any squamates were mobile in this manner, and the fact that they are in at least some groups raises all kinds of questions…. and possibilities if you’re interested in speculative evolution.
Caption: some preserved dibamid specimens are almost orange. However, this is almost certainly because they’ve been sitting in preservative fluid for years or decades, and it likely doesn’t reflect their colour in life. This is the holotype of Dibamus tebal from Pulau Simeuleu (off the south-west coast of Sumatra), named as a new species in 2016 but collected at some point in the 1910s (Das & Lim 2009). The scale bar is in millimetres; the total length is 158 mm. Image: Das & Lim (2009).
Distribution and habitat, part 1. There’s a lot to say about dibamid distribution and what it means for our knowledge of the group. First things first, their disjunct distribution – they occur in eastern Asia, western Australasia and Mexico and nowhere in between – obviously requires an explanation. If this group has a history extending back to the Paleogene or earlier (as it likely does), then maybe this distribution reflects an older, continuous one whereby species formerly occurred across Beringia and North America. If so, we predict the discovery of fossil species in North America and perhaps elsewhere.
On that note, if this group evolved especially early in squamate history, it might even be that they were substantially more widespread in the distant past. An Early Cretaceous or Jurassic origin, for example, could mean a pan-Laurasian distribution, perhaps even a pan-Pangaean one.
An alternative explanation of dibamid distribution is that over-water dispersal occurred, and of course small burrowing reptiles of this sort could conceivably travel out to sea in sediment associated with big floating root masses or clusters of plants. Townsend et al. (2011) examined dibamid distribution and proposed that dibamids crossed the Beringian landbridge during the Paleocene or Eocene, but did also consider the possibility of trans-Pacific rafting. Townsend et al. (2011) also found Dibamus to be paraphyletic, with one set of species being closer to Anelytropsis than to other Dibamus species. This implies that Anelytropsis is geologically young within the group, that eastern Asia is the group’s ‘centre of origin’, and that the movement that explains their disjunct distribution was eastwards in relation to the Pacific.
Caption: the area of Nui Chua National Park, Ninh Thuan Province, southern Vietnam, in which Dibamus tropcentr was discovered. This is a hot and dry location, and not the sort of habitat that you might ordinarily associate with dibamids. The holotype was specifically found in a rotten log, in association with termites. Image: Kliukin et al. (2023).
If dibamids have an evolutionary history going back at least 40 million years, we might speculate that they include more diversity – in ecology, behaviour and physiology – than generally thought. I’d thought from prior reading on the group that they were all animals of tropical forest floors, but this is not so: the Asian/Australasian species occur in tropical and subtropical forest environments while the Mexican one appears to be a habitat generalist, occurring in cloud forest, brush-covered, arid mountains, deciduous woodland, arid scrubby plains and elsewhere. And having said that the Asian/Australasian species “occur in tropical and subtropical forest environments”, it’s important to note the diversity of habitats they occur in, since there are species in lowland arid habitats (including dry maritime evergreen forest) and upland forests too.
Incidentally, Darevsky (1992) reported that the holotype of D. greeri was discovered within a mass of soil, overgrown with epiphytes attached to a moss-covered tree, that fell to the ground from a height of about 3 metres. So, this dibamid was living up in a tree. Presumably, the animal had travelled up the tree by climbing through and under the thick moss layer. Worms and fossorial arthropods are also sometimes found in soil attached to trees in the tropics, so this is not a wholly surprising observation.
Caption: Nui Chua Mountain in Nui Chua National Park in southern Vietnam, the type locality for D. deimontis, described this year (Kliukin et al. 2024). The arrow marks the discovery spot. This is an area where mixed montane evergreen forest occurs alongside grassy areas. There are also streams that contain large, root- and moss-covered granite boulders. The dibamids were collected from the boulder surfaces where they were associated with the mosses and roots. Image: Kliukin et al. (2024).
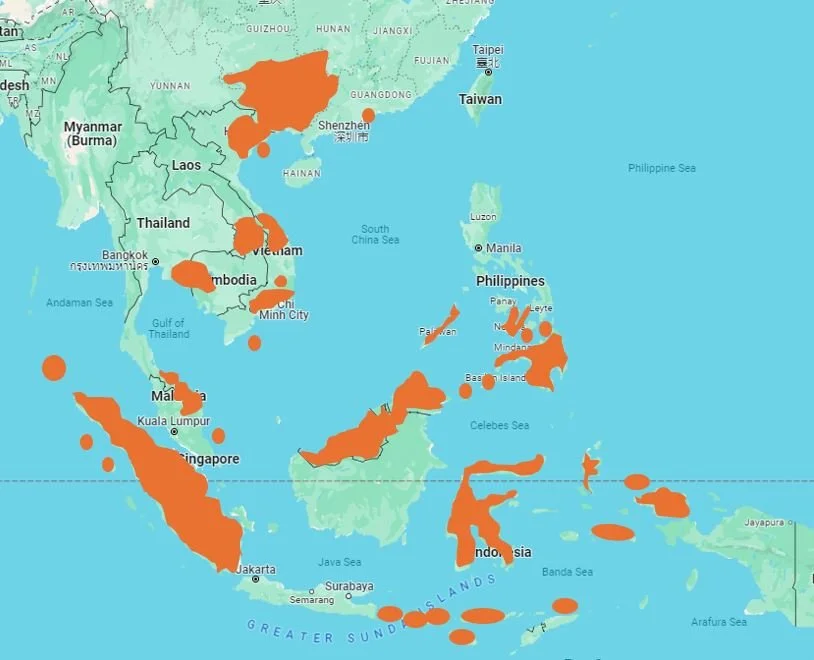
Distribution and habitat, part 2. There’s one more thing I want to say on distribution and habitat, and this is that our scant knowledge of these animals – most species are known from only a handful of specimens – means that the map is quite patchy in terms of where we know they occur. On mainland Asia, the group’s most northerly occurrence is Guangxi Province in southern China, with Hong Kong being their most easterly occurrence. In Southeast Asia, they’re known from the north and south of Vietnam as well as its narrow central section, from south-eastern as well as extreme southern, peninsular Thailand, western and probably northern Cambodia, and the northern half of Malaysia.
As you can see from the map I created (see caption for caveats!), large gaps are present in confirmed dibamid distribution. These gaps suggest that dibamids await discovery in numerous places, including central and southern Vietnam, much of Cambodia, some of eastern and southern Thailand, and probably southern Laos at least. The presence of the group in Tioman Island, adjacent to eastern mainland Malayasia, hints at a presence in southern Malaysia. Certain of the mainland locations from which dibamids are known are (relatively) ‘recent adds’: the 2011 naming of D. dalaiensis from Cambodia, for example, was significant enough that I covered it at Tet Zoo.
Caption: by combing the primary literature on dibamids, I extracted as much locality data on Asian/Australasian dibamids as I could and depicted it on this map. I marked ‘small island’ locations with a spot or oval but attempted to depict ‘large island’ and mainland locations with an extrapolated range. Some caveats are necessary: when several records are known from the same approximate area (e.g., southern Vietnam), I extrapolated a rough range area by ‘connecting the dots’. Records from Sumatra and Sulawesi suggest that dibamids occur across the better part of these islands, but this likely isn’t correct and a more detailed examination would result in far patchier ranges for these regions. Anyway… the key point here is that dibamids remain unknown or scarcely known from many places across the region, and could await discovery in many additional localities.
Islands are important in dibamid distribution. The group’s most westerly Asian occurrence is on the Nicobar Islands, which – politically – are part of India. On the Indonesian islands, dibamids have a known presence (going from west to east) in Sumatra and some surrounding islands, northern Borneo, much of Sulawesi, various of the Lesser Sunda Islands, and Seram. They’re present on some of the southern islands of the Philippines and western New Guinea too. Again, there are lots of gaps here, and I think it’s reasonable to suggest that dibamids might prove more widely distributed in Borneo and might also await discovery on quite a few additional Indonesian islands, including Taliabu, Buru and numerous other of the Maluku Islands, Timor, and Java.
At the moment, the majority of Asian dibamid species occur on islands. But the fact that several new ones have been named from the Asian mainland in recent years indicates that their continental diversity has been unappreciated and Kliukin et al. (2023, p. 318) recently stated that “it is highly likely that the territory of the mainland Indochina, including Vietnam, cradles a comparable if not higher diversity of Dibamus lizards”. They ended their text by emphasizing that more sampling is needed in appropriate habitats in nations like Vietnam.
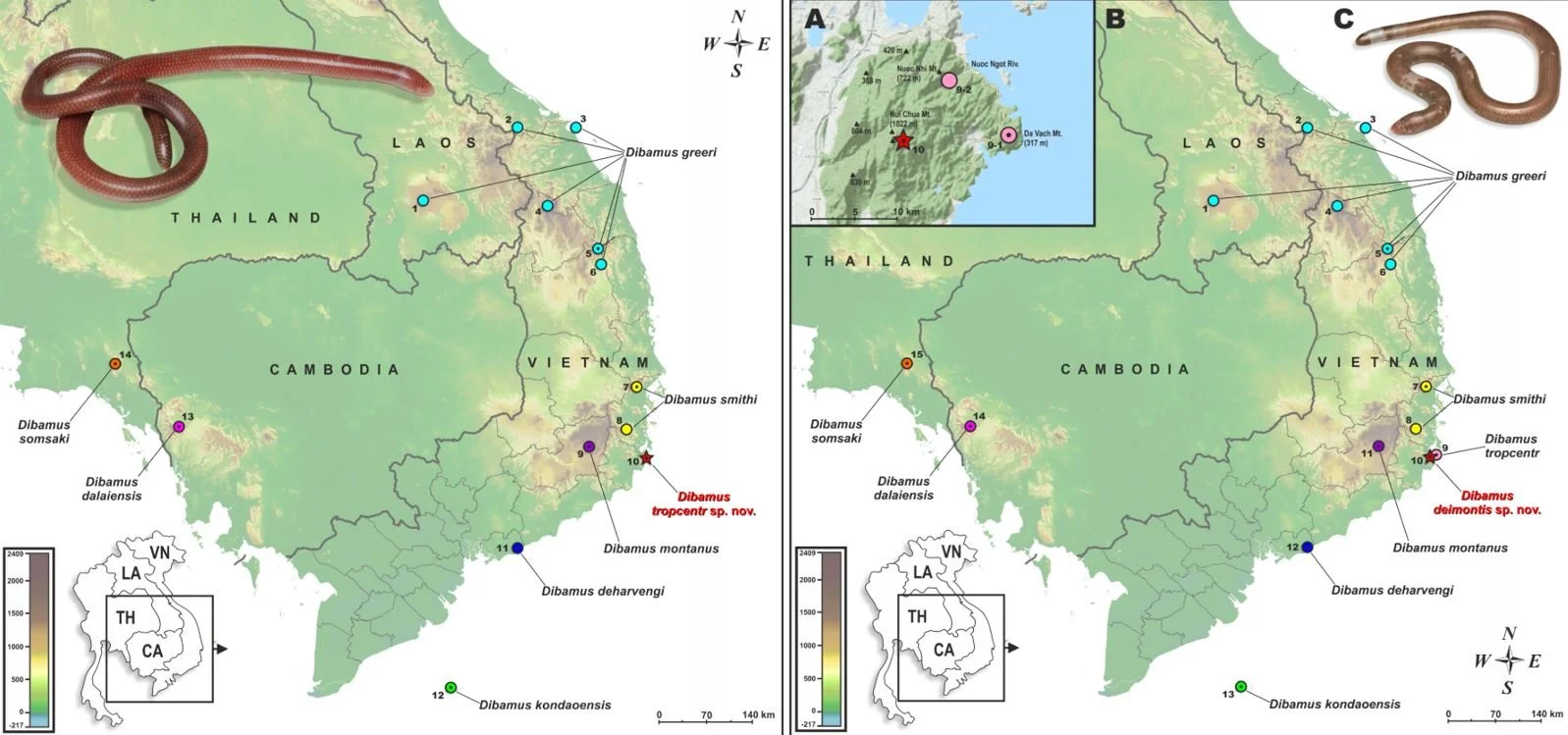
Caption: maps from recently published descriptions of Asian dibamid species, showing locations relevant to the discovery of species from eastern Southeast Asia. The map on the left is from Kliukin et al. (2023), the description of Dibamus tropcentr from eastern Vietnam; that on the right is from the description of D. deimontis Kliukin et al., 2024 from the same area. The publication of D. deimontis takes us to 26 recognized species. Images: Kliukin et al. (2023), Kliukin et al. (2024).
While we’re here… similar comments could be made about the distribution of Anelytropsis in Mexico. Its current distribution is also patchy, there being a large gap between the type locality in central Veracruz and its other occurrences in southern Tamaulipas, San Luis Potosí, and northern Guanajuato and Querétaro. Based on its ecological flexibility, some authors of decades past suggest that it might await discovery further west and south. When Axtell (1958) reported the most northerly known occurrence – at Ciudad Victoria in Tamaulipas – he noted that this might be indicative of “a more widespread range at a time (early Pleistocene?) when humid conditions were more pronounced” (Axtell 1958, p. 189). More record records indicate that this view of a wider Mexican distribution is likely correct: Valdez-Vilavicencio et al. (2016) reported occurrences in Oaxaca in southern Mexico, and Montiel-Veranza et al. (2022) described the presence of the species in Comapa in Veracruz, which is further to the east in Veracruz than previous records.
Caption: images of Anelytropsis from recently published range extension records. At left, an orange specimen from Oaxaca in southern Mexico, reported by Valdez-Vilavicencio et al. (2016) (again, the specimen had been kept in preservative for some years prior to 2016, so this colour might not represent the condition in life); at right, a pinker one from Comapa in Veracruz, reported by Montiel-Veranza et al. (2022).
And… where do dibamids fit in phylogeny? Moving now away from distribution and biogeography, where do dibamids fit in the squamate family tree? I was planning to cover that issue in this article but there’s enough to say about it that I’m going to postpone coverage and return to it later. The spoiler is that dibamids have proved enigmatic, with different authors proposing quite different phylogenetic positions for the group, and with several pushing the hypothesis that dibamids might link amphisbaenians and snakes and prove important in models of snake origins (this being one of the most contentious issues within the field of squamate evolution). Some authors regard dibamids as a highly distinct, ancient lineage within Squamata, and it’s mostly for that reason that the name Dibamia – it implies a ‘higher’ taxonomic status than the ‘family-level’ Dibamidae – has been used by some authors. We will return to these topics in time…
Incidentally, I promised back in 2011 that “much more on dibamids” would appear at “some other time”, so never let it be said that I don’t keep my promises. Sorta.
For previous articles on squamates, see…
Cambodia: now with dibamids!, May 2011
The Tet Zoo Guide to Mastigures, August 2018
The Remarkable Basilisks, May 2023
Do Lizards Really Have ‘Mite Pockets’?, March 2024
Meeting Lake Zacapu’s Garter Snake, March 2024
Refs - -
Axtel, R. W. 1958. A northward range extension for the lizard Anelytropsis papillosis, with notes on the distribution and habits of several other Mexican lizards. Herpetologica 14, 189-191.
Darevsky, I. S. 1992. Two new species of the worm-like lizard Dibamus (Sauria: Dibamidae) with remarks on the distribution and ecology of Dibamus in Vietnam. Asiatic Herpetological Research 4, 1-12.
Das, I. & Lim, K. K. 2009. A new species of Dibamus (Squamata: Dibamidae) from Pulau Simeuleu, Mentawai Archipelago, Indonesia. Zootaxa 2088, 15-23.
Diaz, R. E., Leong, T. M., Grismer, L. L. & Yaakob, N. S. 2004. A new species of Dibamus (Squamata: Dibamidae) from West Malaysia. Asiatic Herpetological Research 10, 1-7.
Greer, A. E. 1982. 1985. The relationships of the lizard genera Anelytropsis and Dibamus. Journal of Herpetology 19, 116-156.
Hallermann, J. 1998. The ethmoidal region of Dibamus taylori (Squamata: Dibamidae), with a phylogenetic hypothesis on dibamid relationships within Squamata. Zoological Journal of the Linnean Society 122, 385-426.
Kliukin, N. S., Bragin, A. M., Nguyen, T. V., Le, S. X., Tran, T. T. V., Gorin, V. A. & Poyarkov, N. A. 2024. Another new species of Dibamus Duméril & Bibron, 1839 (Squamata: Dibamidae) from Nui Chua National Park, Ninh Thuan Province, Vietnam. Zootaxa 5406, 87-104.
Kliukin, N. S., Nguyen, T. V., Le, S. X., Bragin, A. M., Tran, V. T., Gorin, V. A. & Poyarkov, N. A. 2023. A new species of the genus Dibamus Duméril & Bibron, 1839 (Squamata: Dibamidae) from the driest and hottest place of Vietnam. Zootaxa 5380, 301-320.
Koppetsch, T., Böhme, W. & Koch, A. 2019. A new species of Dibamus Duméril & Bibron, 1839 (Squamata: Dibamidae) from Pulau Manado Tua, Northern Sulawesi, Indonesia. Zootaxa 4555, 331-345.
Montiel-Veranza, J. A., Vásquez-Cruz, V. & Morales-Leal, I. 2022. New records of Anelytropsis papillosus Cope, 1885 (Squamata: Dibamidae) for the Municipality of Comapa, in the central-western of Veracruz, Mexico. Revista Latinoamericana de Herpeología 5, 139-141.
Quah, E. S. H., Shahrul Anuar M. S., Grismer, L. L. & Grassby-Lewis, R. 2017. A new species of Dibamus Duméril & Bibron 1839 (Squamata: Dibamidae) from a hill station in Peninsular Malaysia. Raffles Bulletin of Zoology 65, 681-690.
Rieppel, O. 1984. The cranial morphology of the fossorial lizard genus Dibamus Dumeril & Bibron, with a consideration of its phylogenetic relationships. Journal of Zoology 204, 289-322.
Townsend, T. M., Leavitt, D. H. & Reeder, T. W. 2011. Intercontinental dispersal by a microendemic burrowing reptile (Dibamidae). Proceedings of the Royal Society B: Biological Science 278, 2568-2574.
Valdez-Villavicencio, J., Garcia-Padilla, E. & Mata-Silva, V. 2016. Anelytropsis papillosus Cope, 1885 (Squamata: Dibamidae), an overlooked species in the state of Oaxaca, Mexico. Mesoamerican Herpetology 3, 178-180.
Whitfield, P. 1983. Reptiles and Amphibians: An Authoritative and Illustrated Guide. Longman Group Ltd, Harlow, UK.