Another belated book review! Yes, let’s look at…
Predation and Corpse-Eating in Armadillos
The Shrews of the World
Cloudrunners and Other Cloud Rats of the Philippines
I’ve surely said on several occasions over the years that I’ve never written enough about rodents here at TetZoo. But, then, you could write about nothing BUT rodents and still not write about them enough… there are just so many of them, both in terms of numbers of species and individuals. Whatever, I’ve opted today to write about cloudrunners and other cloud rats, a group of luxuriantly furred, large, striking members of Muridae – the rat and mouse family – endemic to the Philippines.
Extreme Cetaceans, Part 3
Hello faithful and noble readers. Recall the unfinished series on EXTREME CETACEANS? Today we continue with the next episode in said series.
Caption: Stenella longirostris, Phocoena dioptrica and Sousa chinensis, three of the cetacean species covered in the previous parts of this series. Image: Darren Naish.
If you don’t know what the deal is here, it’s that I’m writing about those cetaceans which I consider ‘extreme’, this meaning that they’re “weird, possessing anatomical specialisations and peculiarities that are counter-intuitive and little discussed, and most likely related to an unusual ecology, physiological regime, feeding strategy or social or sexual life”, to quote the first article in the series. And thus we get on with it…
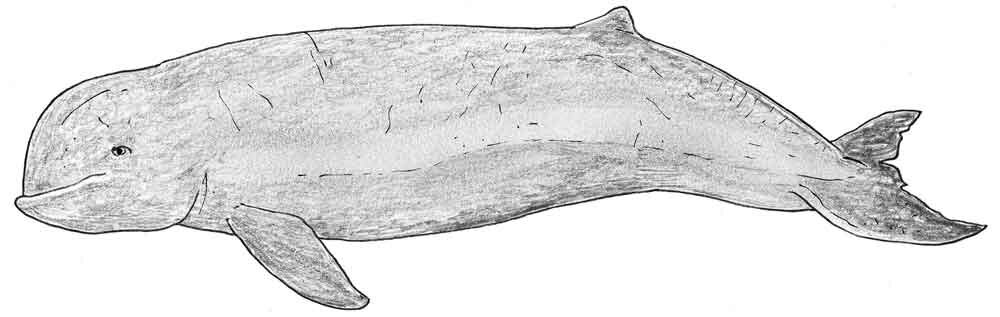
Right whale dolphins. Many dolphin species are aesthetically pleasing because they’re of a beautifully streamlined, attenuate shape, and because they have clean, tidy colour schemes where contrasting blocks of colour are neatly separated, and sometimes augmented or marked by parallel, sweeping lines. This combination – an attenuate, streamlined form and a tidy, well-demarcated colour scheme – is carried to an extreme in the two Lissodelphis species, or right whale dolphins.
Caption: Alcide Dessalines d'Orbigny’s 1847 illustration of the Southern right whale dolphin Lissodelphis peronii. The species is named for naturalist François Peron, the first European to report a sighting of this species. Image: public domain (original here).
Right whale dolphins are mid-sized as dolphins go (about 2-3 m long), short-beaked, and incredibly attenuate. Their pectoral flippers and tail flukes are small, a dorsal fin is absent, and the tailstock tapers to a ridiculous degree. They also have the flashiest, tidiest colour scheme of black and white. They look nothing like the enormous, super-bulky right whales, but do resemble them in lacking a dorsal fin. They’re also incredibly fast, among the fastest of all cetaceans,
Caption: a Southern right whale dolphin group, photographed in 2008. These dolphins are often seen in large groups of 100 individuals or more. Image: Lieutenant Elizabeth Crapo, NOAA Corp, public domain (original here).
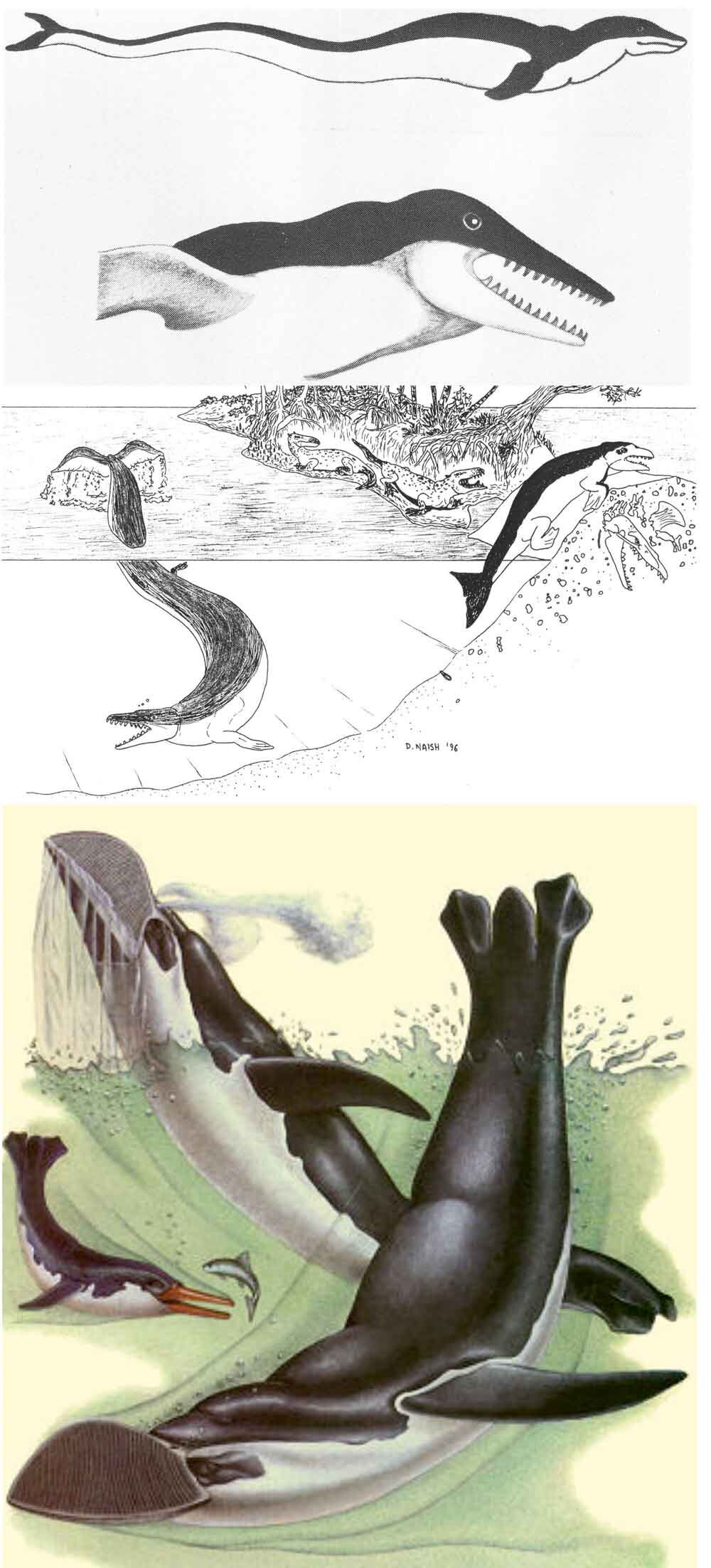
Right whale dolphins, incidentally, are close kin of lags (the Lagenorhynchus and Sagmatias dolphins) and probably of the small, short-beaked Cephalorhynchus dolphins (the most familiar of which is the piebald Commerson’s dolphin C. commersoni) (McGowen et al. 2009). But in my headcanon they’re either miniaturised, late-surviving basilosaurids, or whale-mimicking, fully aquatic penguins that have time-travelled from the Dixonian Era to the present. Look at the pictures here and you’ll see what I mean.
Caption: old depictions of basilosaurs and other archaeocetes – those at top are from McEwan (1978) and Naish (1996) – reveal that right whale dolphins are actually descendants of a lineage outside of Neoceti. Or perhaps they’re future penguins, like the Vortex (from Dixon 1981). Images: McEwan (1978) and Naish (1996), Dixon (1981).
The Pesut. In 1989, I thought I knew all the extant cetacean species known to science at the time. So I was blown away when the Today newspaper, which I used to read, ran a two-page feature on a very odd cetacean which was touted as “the only new breed to be discovered in thirty-four years”, this being a reference to the number of years that had elapsed since the scientific naming of Fraser’s dolphin Lagenodelphis hosei in 1956. Evidently, the article was reporting a proposal – seemingly originating with Francois-Xavier Pelletier – in which the cetacean concerned was being considered a potential new species. Grey, toothless and prone to squirting jets of water for fun, it was said to be a freshwater inhabitant of Borneo’s Mahakam River, and was dubbed the Pesut. The what?
Caption: a Today newspaper article of 1989 reports ‘the Pesut’ as a new kind of dolphin. I regret that I don’t have the complete citation for this article; in my wisdom I clipped the date and other details at some point. Readers with exceptional memories might recognise the photo at upper right as the inspiration for a SpecZoo-themed piece of art…
Today, the Pesut isn’t regarded as a distinct species, but a local variant of the Irrawaddy dolphin Orcaella brevirostris. It’s known locally as the Pesut Mahakam, more formally as the Mahakam River dolphin, and is seemingly – with the rest of the Orcaella dolphins – an early-diverging member of the globicephaline clade (McGowen et al. 2009, Vilstrup et al. 2011), otherwise known for including killer whales, pilot whales and kin, the ‘blackfish’ [UPDATE: killer whales no longer appear to be part of Globicephalinae; see comments]. Pelletier’s proposal that the Mahakam River Orcaella population might be distinct is odd, since anyone familiar with the historical taxonomy of Orcaella knows (or should have known, even in 1989) that Pesut Mahakam is a local name for some riverine populatons of O. brevirostris (Marsh et al. 1989). Furthermore, there’s a long history of riverine Orcaella populations being considered distinct and of having their taxonomic status tested and re-evaluated.
Caption: an Irrawaddy dolphin photographed in Cambodia. Image: Stefan Brending, CC BY-SA 3.0 (original here).
Whatever, the Pesut does look kinda unusual. Books on whales very often say or imply that the Boto or Amazon river dolphin Inia geoffrensis and Beluga Delphinapterus leucas are the only two living cetaceans with an especially mobile neck, but this very probably isn’t true and Pesuts are often shown with the head being held at an obvious angle relative to the body. Other weird features that make the Pesut ‘extreme’ are its globular, short-snouted face and smiling mouthline, and the crease that runs along part of its dorsal midline.
Caption: an effort to portray an Irrawaddy dolphin in life. This dolphin can reach 2.75 m in length, males being larger. Image: Darren Naish.
If you know anything about cetaceans you’ll be aware of the fact that the Irrawaddy dolphin is superficially similar to the Beluga, and it’s this similarity which has led to the occasional suggestion that Orcaella might not be a dolphin but a tropical member of the same family as the Beluga (Monodontidae). This isn’t a ridiculous idea, but it isn’t supported by the detailed anatomy of this animal, or by molecular data.
Caption: I said the montage would become increasingly cluttered. And we’re not done yet. Image: Darren Naish.
And that’s where we’ll end things for now; the next article in the series will appear soon. And I’ll publish a lot more on whales here in the future. Here’s some of the stuff that exists in the archives (as always, much of the material at TetZoo versions 2 and 3 has been ruined by the removal of images, so I’m linking to wayback machine versions)…
A 6 ton model, and a baby that puts on 90 kg a day: rorquals part I, October 2006
From cigar to elongated, bloated tadpole: rorquals part II, October 2006
Lunging is expensive, jaws can be noisy, and what’s with the asymmetry? Rorquals part III, October 2006
On identifying a dolphin skull, July 2008
Seriously frickin' weird cetacean skulls: Kogia, shark-mouthed horror, July 2008
Scaphokogia!, July 2008
Cetacean Heresies: How the Chromatic Truthometer Busts the Monochromatic Paradigm, April 2015
Whale Watching in the Bay of Biscay, August 2019
Extreme Cetaceans, Part 1, September 2019
Extreme Cetaceans, Part 2, September 2019
Refs - -
Dixon, D. 1981. After Man: A Zoology of the Future. Granada, London.
Marsh, H., Lloze, R., Heinsohn, G. E. & Kasuya, T. 1989. Irrawady dolphin Orcaella brevirostris (Gray, 1866). In Ridgway, S. H. & Harrison, R. (eds) Handbook of Marine Mammals Volume 4. Academic Press (London), pp. 101-118.
McGowen, M. R., Spaulding, M., Gatesy, J. 2009. Divergence date estimation and a comprehensive molecular tree of extant cetaceans. Molecular Phylogenetics and Evolution 53, 891-906.
Naish, D. 1996. Ancient whales, sea serpents and nessies part 2: theorising on survival. Animals & Men 10, 13-21.
Vilstrup, J. T., Ho, S. Y., Foote, A. D., Morin, P. A., Kreb, D., Krützen, M., Parra, G. J., Robertson, K. M., de Stephanis, R., Verborgh, P., Willerslev, E., Orlando, L. & Gilbert, M. T. P. 2011. Mitogenomic phylogenetic analyses of the Delphinidae with an emphasis on the Globicephalinae. BMC Evolutionary Biology 11: 65.
Extreme Cetaceans, Part 2
Recall the recent article about ‘extreme cetaceans’? Well, here’s the second one in the series.
Spectacled porpoise. Porpoises – the seven* species of the delphinoid family Phocoenidae – are small, short-beaked cetaceans that mostly live fairly cryptic lives in shallow coastal seas (this description applies to the living species: some fossil porpoises were comparatively large and long-beaked). The species that typifies the group – the Harbour porpoise Phocoena phocoena – is greyish (except for its white belly), has a low, triangular dorsal fin and is not especially charismatic.
* I’ve followed recent taxonomic decisions and am recognising two species within Neophocaena (N. phocaenoides and N. asiaeorientalis).
Caption: Phocoena phocoena, the archtypical member of Phocoenidae. Image: Erik Christensen, CC BY-SA 3.0 (original here).
But other porpoises are rather different, and here we’re going to look at a far more flamboyant species, namely the Spectacled porpoise P. dioptrica of the cool and cold waters of the sub-Antarctic and Antarctic seas. This is a very poorly known species, and one of the things said about it most often is that just about nothing is known about it. It’s a 20th century discovery, its scientific debut occurring in 1912.
This species is remarkably pigmented relative to other Phocoena porpoises, being black dorsally, white ventrally, and with dark circles around its eyes. There have actually been a bunch of competing ideas on its exact appearance over the years, authors and artists disagreeing with respect to where the boundary between its dark and white areas are, what colour its flippers and tail flukes are, and so on. It’s distinct enough from the other Phocoena species that some authors have preferred to keep it in its own genus (Australophocoena), but this isn’t fashionable at the moment due to molecular data on its phylogenetic position. The suggestion has even been made that its pattern and colouring give it the ability to mimic killer whales and thus avoid predation. Cool idea, buuuut…. unlikely given that porpoises are so distinct from killer whales in size and surely in vocalisations and in the echolocatory signature that predatory cetaceans use when evaluating potential prey.
Caption: Spectacled porpoises photographed in the wild, in the Southern Ocean, in 2001. A male is at back, an adult female is closest to us, and a calf is in the middle. Image: Sekiguchi et al. (2006).
The Spectacled porpoise isn’t just remarkable for its pigmentation, however, but also for its shape, and in particular for its dorsal fin. This is ‘normal’ in some individuals, but disproportionally large – strangely so – in some individuals where it looks like an out-sized rounded flag projecting upwards and backwards at a size about twice or three times that you might predict. Like the keels, humps and unusual dorsal fins of some spinner dolphins (see the previous article in this series), this is a sexually dimorphic feature that’s especially exaggerated in mature males. Its presence is therefore presumably a sociosexual indicator of age and sexual status. Another odd thing about the dorsal fin (albeit one not unique to this species within porpoises as a whole) is that there are tiny tubercles along the leading edge (Evans et al. 2001), albeit seemingly not in all individuals. Dorsal fin tubercles are actually known for all porpoises – they’re weird and interesting and I’ll try to remember to come back to them in another article.
Caption: male, female and juvenile Spectacled porpoise, as illustrated by Uko Gorter for Natalie et al. (2018). The remarkable size of the male’s dorsal fin is obvious. Image: (c) Uko Gorter/Natalie et al. (2018).
This giant dorsal fin isn’t a newly discovered feature – it was reported and illustrated as far back as 1916 (Bruch 1916) – but it hasn’t ben commented upon as often as it might, especially given that it’s one of the most pronounced expressions of sexual dimorphism in cetaceans. Indeed, as Ellis (1983) noted, “only the killer whale manifests such a difference in the dorsal fin” (p. 198); sexual dimorphism of the dorsal fin is known in other porpoises, but isn’t as extreme as it is here (Torre et al. 2014). Apart from the fact that it’s obvious, and looks fairly absurd in the older males that have it, we don’t know much about this fin or its function. Maybe it’s ‘just’ a visual signal of sex, maturity and (perhaps) health and condition. Maybe – recall the comments in the previous article about dorsal fins functioning as thermal windows – it also plays an important physiological role. Whatever it does, it makes this an ‘extreme’ cetacean; an animal that looks surprising, weird and flamboyant.
Caption: I’m going to build a montage of the extreme cetaceans discussed in this series. This image will become more cluttered over time. Image: Darren Naish.
Finally — there’s a adoption scheme for the Spectacled porpoise. Adopt one yourself and aid in the conservation of this poorly known species.
More in this series soon. Here’s some of the stuff on cetaceans that exists in the archives (as always, much of the material at TetZoo versions 2 and 3 has been ruined by the removal of images, though remember that much or all of this is archived at Wayback Machine)…
A 6 ton model, and a baby that puts on 90 kg a day: rorquals part I, October 2006
From cigar to elongated, bloated tadpole: rorquals part II, October 2006
Lunging is expensive, jaws can be noisy, and what’s with the asymmetry? Rorquals part III, October 2006
On identifying a dolphin skull, July 2008 (all images now missing)
Seriously frickin' weird cetacean skulls: Kogia, shark-mouthed horror, July 2008 (all images now missing)
Scaphokogia!, July 2008 (all images now missing)
Cetacean Heresies: How the Chromatic Truthometer Busts the Monochromatic Paradigm, April 2015 (but now lacking all images)
Whale Watching in the Bay of Biscay, August 2019
Extreme Cetaceans, Part 1, September 2019
Refs - -
Bruch, C. 1916. El macho de Phocaena dioptrica Lah. Physis, 2461-2462.
Ellis, R. 1983. Dolphins and Porpoises. Robert Hale, London.
Evans, K., Kemper, C. & Hill, M. 2001. First records of the Spectacled porpoise Phocoena dioptrica in continental Australian waters. Marine Mammal Science 17, 161-170.
Natalie, R., Goodall, P. & Brownell, R. L. 2018. Spectacled Porpoise. In Würsig, B., Thewissen, J B. M. & Kovacs, K. (eds) Encyclopedia of Marine Mammals, Academic Press, pp. 912-916.
Sekiguchi, K., Olavarría, C., Morse, L., Olson, P., Ensor, P., Matsuoka, K., Pitman, R., Findlay, K. & Gorter, U. 2006. The spectacled porpoise (Phocoena dioptrica) in Antarctic waters. Journal of Cetacean Research and Management 8, 265-271.
Torre, J., Vidal, O. & Brownell, R. L. 2014. Sexual dimorphism and developmental patterns in the external morphology of the vaquita, Phocoena sinus. Marine Mammal Science 30, 1285-1296.
Whale Watching in the Bay of Biscay
Back in July 2019, myself and a bunch of friends stepped aboard the Pont-Aven for several days of sea-watching in the Bay of Biscay. We were to travel from Plymouth (UK) to Santander (Spain), the event being organised by ORCA, a charity that monitors whales and uses the data for conservation purposes (they’re here on Twitter). ORCA uses cruise liners, ferries and other vehicles as whale-watching platforms. Nigel Marven was a special guest on our trip and it was great to catch up with him.
Caption: our vessel of choice - the Pont-Aven - at port in Santander, Spain. I cannot tell you how much trouble I went to to get to this ship before departure time. I very nearly didn’t make it. Image: Darren Naish.
Caption: the man, the legend; Nigel Marven.
The purpose, of course, was to see whales. The weather was outstandingly good (meaning that I got burnt), but so was the whale watching: I’m pleased to say that we saw literally hundreds of animals of seven or eight species, as you can see from the photos below. My own photos are not great since my camera isn’t exactly the best for fast-moving, far-away animals like whales, so those you see here were mostly taken by my trusty pal Alex Srdic (who’s here on Instagram and here on Twitter). Thanks, Alex.
Caption: several cetaceans have extremely complex markings allowing them to be identified to species and even population. Individuals can be recognised on the basis of their markings too. Image: Alex Srdic.
The Bay of Biscay is a world-famous whale-watching hotspot, famous in particular for Cuvier’s beaked whales Ziphius cavirostris and Sperm whale Physeter macrocephalus. Dolphins of several species are a frequent sight too, as are rorquals of a few species, Harbour porpoise Phocoena phocoena and pilot whales. A very lucky whale-watcher might get to see Blue whale Balaenoptera musculus, Killer whale Orcinus orca or True’s beaked whale Mesoplodon mirus. In fact, something like 30 species have been recorded in the region. This is phenomenal and mean that it’s theoretically possible for several species of some of the most elusive whale groups – like beaked whales and globicephaline dolphins – to be seen within days or weeks of each other.
Caption: in good weather, the blow of a big whale (like a Fin whale - as here - or a Sperm whale) is visible from great distance, and in the case of these two species can be diagnostic. Image: Alex Srdic.
Caption: a dynamic leap by a Striped dolphin. Dolphins of some species appear to be attracted to ships and even to deliberately show off when they get close to them. Image: Alex Srdic.
Why is the Bay of Biscay so good for whales? It’s mostly because the topography is complex, combining large, shallow shelf regions, steep sections of shelf edge – sometimes with impressive slopes and deep, enormous rocky canyons twice as big as the Grand Canyon – and a deep abyssal plain section (Carwardine 2016). Depth varies from 1.7 to over 4.7 km. This variation – combined with the overall productivity of the region and its position relative to the Atlantic and English Channel – means that there’s the chance to see continental shelf species (like porpoises), those that use deep canyons and other shelf-edge habitats (like beaked whales) and true oceanic deep-divers that forage in the deepest waters (like sperm whales).
Caption: back and dorsal fin of a Fin whale, remnants of the blow still hanging in the air. Image: Alex Srdic.
As it happens, we were extraordinarily lucky. Fin whales B. physalus are regular animals of the area, and we had amazing, relatively close views of them (by ‘close’, I mean perhaps 30 m from the ship, not alongside the vessel). Fin whales – the second largest extant animal after the Blue – have a blow that’s visible on the horizon and is about 8 m tall. The blow hangs in the air for a surprising time. One of the most remarkable things about the Fin whale is its asymmetrical pigmentation: the right side of the face is marked with a large pale area, as is the right side’s baleen. There are some old TetZoo articles on what this might mean and how it might function – see the links below.
Caption: excellent view of the splashguard - the conical structure surrounding and ahead of the blowholes - and paired blowholes of a surfacing Fin whale. Despite its name, the dorsal fin of the Fin whale is smaller and blunter than that of some other rorquals. Image: Alex Srdic.
Two coastal species were seen early on in our trip: Harbour porpoise and Common bottlenose dolphins Tursiops truncatus, though I don’t have good photos of either. The majority of dolphins seen on our trip (as is typical for Biscay whale watching) were Short-beaked common dolphin Delphinus delphis, which were sometimes seen in groups of more than ten. Their distinctive hourglass markings are always visible when they leap – which they often do, sometimes while immediately adjacent to a ship – and we also got to see calves on one or two occasions.
Caption: here’s the whole-body view of the common dolphin shown in detail above. This individual only has one stripe extending from the beak to the flipper, with a large pale area separating the eye and flipper. Different configurations are present in different populations. Image: Alex Srdic.
Caption: as the light begins to fade during the later part of the day, a group of Short-beaked common dolphin carve through a surging wave. Note the calf close to the adult at upper right. Image: Alex Srdic.
We also had excellent views of Striped dolphin Stenella coeruleoalba. They behaved in characteristic acrobatic fashion, leaping high out of the water, making impressive splashes and jumping in the ship’s wake. They typically make a lot more disturbance at the water’s surface than do common dolphins, creating great bursts of spray and rooster-tail patterns when they leap and surge. Striped dolphins are near-globally distributed. They’ve been the source of discussion lately since it’s recently been shown that the Clymene dolphin S. clymene is a naturally occurring hybrid between this species and the Spinner S. longirostris (Amaral et al. 2014).
Caption: we had many excellent views of high-leaping Striped dolphin. Note how much spray and splashing is associated with the leaping of this species. Image: Alex Srdic.
Finally as goes dolphins, we also saw pilot whales, identified on the basis of their black colouration and strongly backswept dorsal fins. These were most likely Long-finned pilots Globicephala melas (it’s more typical of temperate and cold waters than the Short-finned G. macrorhynchus) but we didn’t see any of the key features that allow the two species to be distinguished, and none of our photos are good enough to warrant sharing. A mysterious whale was seen among the pilot whales. It seemed to be very dark and with a short, blunt-tipped, parallel-sided but only weakly curved dorsal fin; I don’t think that its head was seen but I had the impression that it was a shallower-bodied animal than the pilot whales. Several different views were offered on its identity with the most likely (on the basis of dorsal fin shape) being that it was perhaps a False killer whale Pseudorca crassidens. That’s not tremendously likely but not impossible.
The whale most famously associated with the Bay of Biscay is Cuvier’s beaked whale, seen so frequently in the area that it’s regarded as the premier location for sightings of this species, worldwide. I don’t know if you’re guaranteed a sighting of a Cuvier’s while there, but – whatever – we were lucky, since we saw nearly 20 of them, ranging from smooth, clean-bodied youngsters to heavily scarred males.
Caption: Cuvier’s beaked whale, seen relatively close to the ship. Image: Alex Srdic.
Caption: heavily scarred Cuvier’s beaked whale, seen at distance and only briefly. We didn’t see any other individuals with scarring as impressive as this. Image: Alex Srdic.
Some individuals have markedly pale heads sharply demarcated from the rest of the body, others do not. On occasion, one or two individuals were close enough to the ship that I was able to get a half-decent shot with my mobile phone. Each sighting was a huge thrill. While we were oh so lucky as goes Cuvier’s, we didn’t see sperm whale, alas. We also saw Northern minke B. acutorostrata on perhaps two occasions, though again I don’t have any good photos.
Caption: another plus… amazing sunsets, and sunrises too. Image: Darren Naish.
Finally, we didn’t just see whales. The same route is also great for seabirds, and we also saw such fishes as tunas and sunfishes. As much as I’d like to start talking about the birds, I’m out of time. Anyway – the trip was excellent: rewarding, fun, and educational. I’ll definitely be doing it again. You should consider supporting ORCA and their work as well.
Cetaceans have been covered at length on TetZoo before - mostly at ver 2 and ver 3 - but these articles are now all but useless since all of their images have been removed (and/or they’re paywalled, thanks SciAm). Over time, I aim to build up a large number of cetacean-themed articles here at ver 4.
A 6 ton model, and a baby that puts on 90 kg a day: rorquals part I, October 2006
From cigar to elongated, bloated tadpole: rorquals part II, October 2006
Lunging is expensive, jaws can be noisy, and what’s with the asymmetry? Rorquals part III, October 2006
On identifying a dolphin skull, July 2008 (all images now missing)
Seriously frickin' weird cetacean skulls: Kogia, shark-mouthed horror, July 2008 (all images now missing)
Scaphokogia!, July 2008 (all images now missing)
Cetacean Heresies: How the Chromatic Truthometer Busts the Monochromatic Paradigm, April 2015 (but now lacking all images)
Refs - -
The New World Leaf-Nosed Bat Radiation
I’ve said a few times here at TetZoo that bats have never really been given adequate coverage. This isn’t because I’m not interested in them: on the contrary, I think about bats more than I think about most other groups of mammals, and I see them and watch them more often than I do most other mammal groups. For a group that includes about 18% of extant mammalian species (using 2019 figures*), I can’t pretend to have ever given bats fair coverage. Having said all that, bats have actually been covered at TetZoo a fair bit: there was an entire 20-part series on vesper bats (properly Vespertilionidae) at ver 3, and I also published several ver 2 articles on the history and evolution of vampire bats, and on much else besides. The fact that all of these articles have been rendered worthless via the removal of their images is mightily dispiriting though, and essentially means that I need to start from scratch.
* c 6495 mammal species, c 1200 bat species.
Caption: TetZoo Towers bat library. The several boxfiles of reprints and photocopied articles are not shown. Image: Darren Naish.
Here, I want to talk about a group I don’t think I’ve ever covered at TetZoo before, namely the phyllostomids, or New World leaf-nosed bats, American leaf-nosed bats or spear-nosed bats. This is a large, American group that contains around 200 living species, making it the third largest bat family (vesper bats are the biggest group, followed by fruit bats). The group has sometimes been called Phyllostomatidae – the vernacular version of which is phyllostomatid – but this is less popular than Phyllostomidae. I have no idea which is really correct here and opt to merely follow majority usage on these sorts of things (insert quote from Gene Gaffney**). It’s not strictly true that I’ve never covered phyllostomids before, since vampires – once upon a time given their own eponymous family (Desmodontidae) – are now universally agreed to be nested within Phyllostomidae, and I have at least written about them.
Caption: Chrotopterus, a big spear-nosed bat. Notice how this bat has relatively broad, low-aspect wings and a large, deep uropatagium (the membrane between the legs). Contrast this with some of the images below. Image: George Henry Ford, public domain (original here).
Phyllostomids occur from Argentina in the south to the southern USA (Nevada being their most northerly occurrence) in the north, and they’re highly diverse ecologically and behaviourally. They include insectivores, frugivores, nectarivores, palynivores (that’s pollen-eaters), omnivores, animalivores and (of course) obligate sanguivores. Numerous different taxonomic subdivisions have been named. We don’t need to worry about any of this in detail but, in simplified terms, Macrotinae (big-eared bats), Micronycterinae (little big-eared bats) and Desmodontinae (vampires) are outside a much larger clade that includes Vampyrinae (false vampires and kin) and Phyllostominae (spear-nosed bats and kin) as well as the nectarivorous and frugivorous Glossophaginae (long-tongued and long-nosed bats) and Stenodermatinae (American fruit bats, fig-eating bats and kin) (Baker et al. 1989, 2003, 2012; but see Wetterer et al. 2000). Vampyrinae is a clade within Phyllostominae according to some studies, in which case it gets down-graded to Vampyrini (Baker et al. 2003). All of this is depicted in a cladogram below.
Phyllostomids are mostly brownish bats with simple, narrow ears. A nose-leaf – typically simple and spear-shaped – is common but not present in all species, a tragus is always present, and many (but not all) of the species that lack nose-leaves have chin-leaves (or a series of chin ‘warts’) instead. Facial stripes are common, dark dorsal stripes are present in a few species, and such things as white patches at the wing tips and yellow rims to the ears and nose-leaves are present in some (Hill & Smith 1984). The tail is variously long, or short, and even absent altogether in some taxa, and similar variation is present in the uropatagium, or tail membrane.
Caption: the tail and uropatagia (the membranes joining the inner sides of the legs to the tail) are reduced, and sometimes highly reduced, in some phyllostomids. Here, we see this reduced condition in (at left) the Toltect fruit-eating bat Dermanura tolteca and (at right) in a Little yellow-shouldered bat Sturnira lilium. Images: M.H. de Saussure, 1860, in public domain (original here); Tobusaru, wikipedia CC BY 3.0 (original here).
Skeletally, phyllostomids are robust, have a distinctive humerus where the distal end is angled relative to the shaft, and have a prominent secondary articulation between the large bony lump (properly termed the greater tuberosity) at the proximal end of the humerus and the scapula (Czaplewski et al. 2007). That’s right: a number of bat groups have an accessory peg-in-socket articulation involving the humerus and the body of the scapula. This means that the humerus and scapula are locked together during the upper part of the wing stroke (Hill & Smith 1984).
Caption: the most prominent exception to the ‘phyllostomids are mostly brown’ generalisation is the Honduran white bat Ectophylla alba, sometimes likened to a fuzzy ping-pong ball and well known for its habit of constructing tents by biting through leaf ribs such that the two sides of the leaf droop on either side of the central axis. Note the yellow ears and nose leaf! The individual at left is releasing a bit of urine. Images: Geoff Gallice, wikipedia, CC BY 2.0 (original here); Leyo, wikipedia, CC BY-SA 2.5. (original here)
Some phyllostomids are really exceptional as goes their anatomical and behavioural novelty. Perhaps the most remarkable are the long-tongued glossophagine flower bats, some of which have extraordinary tubular snouts, remarkably long tongues tipped with papillae, and a highly reduced dentition. The most extreme example of this sort of thing is the Banana bat, Trumpet-nosed bat or Colima long-nosed bat Musonycteris harrisoni of Mexico, an ‘extreme’ mammal as goes snout length. It’s fairly typical for people who aren’t that familiar with bat diversity to confuse glossophagines with the Old World flower-feeding megabats grouped together in Macroglossinae. There’s obviously a degree of evolutionary convergence here, though it hasn’t been that well explored in the literature, to my knowledge. Various glossophagines have symbiotic relationships with sympatric plants. Incidentally, Pallas’s long-tongued bat Glossophaga soricina is able to see UV light (Winter et al. 2003).
Caption: some distantly related (but broadly similar) members of the phyllostomid clade Glossophaginae. At left: a long-tongued champion (though not necessarily the longest-tongued of phyllostomids), Pallas’s long-tongued bat Glossophaga soricina. At right: Underwood’s long-tongued bat Hylonycteris underwoodii. Images: Betty Wills, wikipedia CC BY-SA 4.0 (original here); Karin Schneeberger/Felineora, wikipedia CC BY-SA 3.0 (original here).
Entirely different specialisations are seen in the short-faced, frugivorous phyllostomids included within Stenodermatinae. These have flattened, broad teeth, typically have white facial stripes (an aposematic warning of their powerful bites?), and are sometimes handsome or even cute, big-eyed bats. One of the strangest of bats – the Wrinkle-faced or Lattice-winged bat Centurio senex – belongs to this group. The naked, wrinkled faces of males are mostly concealed by massive skin flaps when the bat is roosting or sleeping. There are also neck glands that seem to secrete scent, and obvious transverse bands on the wing membranes.
Caption: resting Wrinkle-faced bats Centurio senex partially conceal their faces beneath thick skin folds. Translucent patches on the lower of these skin folds seem to allow these bats to detect light-level changes even when their faces are covered. Image: Jplevraud, wikipedia CC BY-SA 3.0 (original here).
My favourite phyllostomids are very different from tubular-snouted flower-feeders and short-face fruit-eaters: they are the robust, more generalised species traditionally lumped together in Phyllostominae (though the name Vampyrinae has also been used for some of them). These are mostly omnivores that eat insects, fruit and small vertebrates, and some are specialised predator bats that variously catch and eat amphibians, mammals (including other bats) and birds. They include Peters’s woolly false vampire Chrotopterus auritus, the Frog-eating bat Trachops cirrhosus – famous for eating frogs and selecting them on the basis of their calls – and the spectacular Linnaeus’s false vampire Vampyrum spectrum, a predatory giant that can, in cases, have a wingspan of over 1 meter.
Caption: Vampyrum, the False vampire or Spectral bat (see comments for a hot take on the term ‘false vampire’), has to be considered one of the most awesome of all bats. It’s convergently similar to the distantly related megadermatid bats of Africa, Asia and Australasia, also (confusingly) often called false vampires. Image: Marco Tschapka, wikipedia, CC BY-SA 3.0 (original here).
Caption: the very impressive skull of Vampyrum. It is robust, with big, strong teeth, especially prominent upper canines (which have an additional internal cusp) and a prominent sagittal crest. The skull can be 5.1 cm long in total (which is big for a bat). Image: Naturalis Biodiversity Center, wikipedia, public domain (original here).
When this variation in feeding ecology is mapped onto a phylogeny, it would appear that the earliest phyllostomids were insectivorous, that omnivory, nectarivory (or nectivory, take your pick) and palynivory evolved from among these insectivores, and that frugivores evolved from among nectarivores and palynivores (Baker et al. 2012). The highly specialised vampires appear – according to phylogenetic data – to have evolved directly from insectivores (which is a surprise in view of some models proposed to explain vampire evolution) and at least some members of the main frugivorous clade appear to have reverted to insectivory (Baker et al. 2012; but see Wetterer et al. 2000). Of the various evolutionary events that must have occurred here, it’s the transition to obligate frugivory that seems to have been the most successful, since the frugivorous clade is the largest (as in, most species-rich) within Phyllostomidae, containing about 70 species in 20 genera.
Caption: a few more vertebrate-eating phyllostomids. At left: California leaf-nosed bat Macrotus californicus, the most northerly occurring phyllostomid. At right: Fringe-lipped bat Trachops cirrhosus, a widespread species of Central and South America that eats seeds, fruits, arthropods and lizards in addition to frogs. Images: National Wildlife Service, wikipedia, public domain (original here); Karin Schneeberger/Felineora, wikipedia CC BY 3.0 (original here).
This is also the radiation that’s seemingly resulted in the greatest, most rapidly evolved amount of morphological variation, since everything here seems to have happened within the last 10 million years and has given rise to taxa that are among the most divergent and specialised of phyllostomids. Also of interest here is that some lineages within this frugivorous clade appear to have evolved in the Antilles before invading the mainland (Dávalos 2007), a case of ‘upstream colonisation’ that contradicts traditional scenarios whereby continental animals give rise (via ‘downstream colonisation’) to island-dwelling forms.
Caption: substantially simplified phyllostomid cladogram, based mostly on Baker et al. (2003), and using their nomenclature (though they regarded false vampires - as Vampyrini - as nested within Phyllostominae). Images (top to bottom): Macrotus = National Wildlife Service, wikipedia, public domain (original here); Desmodus = Uwe Schmidt, wikipedia, CC BY-SA 4.0 (original here); Vampyrum = Marco Tschapka, wikipedia, CC BY-SA 3.0 (original here); Phyllostomus = Karin Schneeberger/Felineora, wikipedia, CC BY 3.0 (original here); Platalina = Juan A. Malo de Molina, wikipedia, CC BY-SA 3.0 (original here); Sturnira = Burtonlim, wikipedia, CC BY-SA 3.0 (original here).
Where in the bat tree? What sort of bats are phyllostomids, and what do we know about their evolutionary history? On the basis of anatomical characters, bat experts have generally thought that phyllostomids are close allies of naked-backed, moustached or ghost-faced bats (Mormoopidae) and bulldog bats and kin (Noctilionidae), the whole lot being grouped together in a clade termed either Phyllostomatoidea or Noctilionoidea (and it’s the last of those terms that should be preferred, so I understand). In turn, this group was thought – again, on the basis of anatomical characters – to be closely related both to vesper bats and their kin (Vespertilionoidea), and to a clade that includes both sheath-tailed bats and kin (Emballonuroidea) and horseshoe bats and kin (Rhinolophoidea) (Smith 1976).
Caption: prior to recent (post-2000-ish) molecular studies, noctilionoids were thought to be close kin of rhinolophoids as well as emballonuroids and vespertilionoids. Rhinolophoids are now known to belong elsewhere. The illustrations here are among the many, many bat drawings I’ve done for my in-prep textbook project, progress on which can be seen here. Image: Darren Naish.
Molecular studies, mostly published since 2000, have substantially revised our view of the bat family tree, however, and it’s now clear that rhinolophoids are not close to the other groups listed here at all (they are, instead, close relatives of megabats). Noctilionoids are still close kin of vespertilionoids, however. It also now seems that Mystacinidae and Myzopodidae are part of Noctilionoidea (Jones et al. 2002, 2005, Teeling et al. 2005, 2012). I’ll be talking more about ideas on bat phylogeny in a future article.
Caption: simplified cladogram depicting the affinities of several of the bat groups shown - via morphological and molecular studies - to belong together within Noctilionoidea. The illustrations here are among the many, many bat drawings I’ve done for my in-prep textbook project, progress on which can be seen here. The Vampyrum representing Phyllostomidae, incidentally, is a placeholder which needs replacing (the existing illustration was copied directly from the work of another artist). Image: Darren Naish.
What does the fossil record say about phyllostomid history? The pre-Pleistocene phyllostomid record is not great but it’s still at least good enough to show that the extinct phyllostomids of the Miocene – most notably those from La Venta in Colombia – were superficially much like living ones, and that the extinct species concerned were doing the sorts of things that phyllostomids do today. The group had almost certainly, therefore, undergone its main flowering and diversification by around 20 million year ago. The Pleistocene phyllostomid record, in contrast, is good and numerous extant taxa are known from sediments of this age.
Caption: beautiful illustration of Salvin’s big-eyed bat Chiroderma salvini, a stenodermatine phyllostomid that has a wide range across South and Central America. The facial stripes are not normally this pronounced in life, though it should be noted that populations are variable as goes stripe thickness. Image: Joseph Smit, in public domain (original here).
And that’s where we’ll end things for now. I’d like to say a lot more about these bats, so we’ll be returning to them in time. And in fact I need to say a lot more about bats in general, so stay tuned for that too.
For previous TetZoo articles on bats (concentrating here on articles that haven’t been stripped of images, as is the case for all ver 2 articles and the vast majority of ver 3 articles)…
Chewed bones and bird-eating microbats, June 2006
Greater noctules: specialist predators of migrating passerines, June 2006
We flightless primates (on the Pettigrew ‘megabats are close kin of primates’ model), August 2006
Books of the TetZooniverse: of Palaeoart, Bats, Primates and Crocodylians, August 2015
Fossil Bat Stories, Part 1, February 2018
Fossil Bat Stories, Part 2: What Are Noctilionids? What Are Noctilionoids?, February 2018
Fossil Bat Stories, Part 3: Bulldog Bats, February 2018
Refs - -
Baker, R. J., Bininda-Emonds, O. R. P., Mantilla-Meluk, H., Porter, C. A. & Van Den Bussche, R. A. 2012. Molecular time scale of diversification of feeding strategy and morphology in New World leaf-nosed bats (Phyllostomidae): a phylogenetic perspective. In Gunnell, G. & Simmons, N. (eds). Evolutionary History of Bats: Fossils, Molecules and Morphology. Cambridge University Press, Cambridge, pp. 385-409.
Baker, R. J., Hoofer, S. R., Porter, C. A. & Van Den Bussche, R. A. 2003. Diversification among New World leaf-nosed bats: An evolutionary hypothesis and classification inferred from digenomic congruence of DNA sequence. Occasional Papers, Museum of Texas Tech University 230, 1-32.
Baker, R. J., Hood, C. S. & Honeycutt, R. L. 1989. Phylogenetic relationships and classification of the higher categories of the New World bat family Phyllostomidae. Systematic Zoology 38, 228-238.
Czaplewski, N. J. 1997. Chiroptera. In Kay, R. F., Madden, R. H., Cifelli, R. L. & Flynn, J. J. (eds) Vertebrate Paleontology in the Neotropics: The Miocene Fauna of La Venta, Colombia. Smithsonian Institution Press (Washington and London), pp. 410-431.
Dávalos, L. M. 2007. Short-faced bats (Phyllostomidae: Stenodermatinae): a Caribbean radiation of strict frugivores. Journal of Biogeography 34, 364-375.
Hill, J. E. & Smith, J. D. 1984. Bats: A Natural History. British Museum (Natural History), London.
Jones, K. E., Bininda-Emonds, O. R. P. & Gittleman, J. L. 2005. Bats, clocks, and rocks: diversification patterns in Chiroptera. Evolution 59, 2243-2255.
Jones, K. E., Purvis, A., MacLarnon, A., Bininda-Emonds, O. R. P. & Simmons, N. B. 2002. A phylogenetic supertree of the bats (Mammalia: Chiroptera). Biological Reviews 77, 223-259.
Smith, J. D. 1976. Chiropteran Evolution. Texas Tech University, Lubbock.
Teeling, E. C., Dool, S. & Springer, M. S. 2012. Phylogenies, fossils and functional genes: the evolution of echolocation in bats. In Gunnell, G. & Simmons, N. (eds). Evolutionary History of Bats: Fossils, Molecules and Morphology. Cambridge University Press, Cambridge, pp. 1-22.
Teeling, E. C., Springer, M. S., Madsen, O., Bates, P., O’Brien, P. & Murphy, W. J. 2005. A molecular phylogeny for bats illuminates biogeography and the fossil record. Science 307, 580-584.
Wetterer, A. L., Rockman, M. V. & Simmons, N. B. 2000. Phylogeny of phyllostomid bats (Mammalia: Chiroptera) data from diverse morphological systems, sex chromosomes, and restriction sites. Bulletin of the American Museum of Natural History 248, 1-200.
Winter, Y., López, J. & von Helversen, O. 2003. Ultraviolet vision in a bat. Nature 425, 612-614.
** In a technical article on fossil side-necked turtles, Gaffney said of a very similar nomenclatural disagreement: “it’s true, I don’t give a rat’s ass which is used”.
New Living Animals We Want to Find
As a regular denizen of the TetZooniverse, you may well remember the July 2017 article ‘Fossils We Want to Find’ in which I discussed a list of hypothetical fossil things that we might one day discover but haven’t yet. Wouldn’t it be fun to do the same sort of thing with extant species; that is, with discoveries pertaining to living, breathing animals? Over at the Zoology for Enthusiasts facebook group (a spinoff of the Tetrapod Zoology facebook group), Jordan Fryer suggested doing exactly this, and consequently people have been coming up with their own suggested living animals that might await discovery. Because this seemed like a lot of fun (and a chance to discuss some really neat and unusual stuff), I thought I’d give it a go.
Caption: the 2017 precursor to the article you’re reading here was all about fossil animals. It included this photo, which shows me in the act of discovering a dinosaur bone in the Moroccan Sahara. Image: Richard Hing.
Naturally, any list of this sort is horribly subjective, reflecting the interests and biases of the person compiling the list, but so be it. It also seems all too easy to turn any compilation into a ‘list of most discoverable cryptids’: for those of you who don’t know, I have a long-standing interest in cryptozoology and have published on it quite frequently (see Naish (2017) for starters). For the most part, I’ve not done this, though read on.
Caption: many of my thoughts on mystery animals can be found in my 2017 book Hunting Monsters. I am not - sorry - much impressed by the case for such supposed animals as the mokele-mbembe, an artistic reconstruction of which is shown at right. Image: David Miller, in Mackal (1987).
I’ve also mostly excluded hypothetical discoveries that are inspired by the creatures of cryptozoology but could arguably be considered independent of the cryptozoological literature. In part this is because I don’t think they’re plausible or worth considering, but it’s also because they’re cliched and the opposite of original. So, no ‘living sauropods from the Congo’ or ‘living plesiosaurs in Loch Ness’, for example.
As for what I have selected: well, some of my suggestions are sillier than others, and some are perhaps not that interesting to non-specialists. But, whatever. Feel free to dissect my suggestions in the comments, and perhaps come up with your own.
Caption: among my suggested ‘fossils we want to find’ are protobats (like the hypothetical examples shown at left, from Graham (2002)) and a good skeleton of the giant hominid Gigantopithecus blacki. This ilustration of a lower jaw is from Simons & Ettel's (1970) magazine article. Images: Graham (2002), Simons & Ettel (1970).
A habitually bipedal, large, non-human hominid. Whatever you think of all those stories, anecdotes and sightings about bigfoot, yeti, almas, orang-pendek, yowie and so on and on, the fact remains that the discovery of a large, bipedal non-human hominid – whether it be a pongine, hominine, or member of another hominid lineage – would be a huge deal. It would not just be one of the most newsworthy creatures to ever be discovered; it would also have enormous ramifications for our understanding of hominid evolution and potentially the human condition itself.
Caption: are crypto-hominids a cultural phenomenon more than a zoological one? I’ve argued for both possibilities at different times. Whatever… for the purposes of the article you’re reading now, I hope we can agree that the discovery of such an animal would be high on any hypothetical ‘wants’ list. Image: Darren Naish.
It would also – if relating to North America or northern Eurasia in particular – very likely have a significant impact on economy, land management and land use in those regions… or, you’d hope it would, anyway (who knows, given the current state of environmental protection in the USA). The hypothetical discovery of such an animal would also be regarded by many as one of the biggest ‘wins’ ever scored against ‘establishment science’, and thus could well be a bad thing (viz, “if scientists were wrong about this, what else could they be wrong about?”). And I’ll stop there before we dive into a rabbit-hole of conspiracy theories and coverups.
A big, flightless passerine. The majority of living bird species – over 60% of them – are passerines, or perching birds. This is the great group that includes crows, thrushes, warblers, finches, sparrows and so many others. For all their success, wide distribution and diversity, passerines are generally quite samey. There are no big, long-legged wading passerines, or heavy-bodied diving passerines or flightless running passerines, for example. Why this is so remains mysterious: passerines didn’t take to those niches because… well, they just didn’t. Does this mean that they couldn’t? As usual, we can come up with a few reasons as to why they were ‘constrained’ in evolutionary potential, but any one of those reasons could be overturned by some evolutionary deviant that refuses to pay attention to the rules.
Caption: passerine birds are diverse, to a degree… here’s just a sample of their diversity. This is part of a giant montage that’s being built for my in-prep textbook The Vertebrate Fossil Record. Image: Darren Naish.
And thus I submit that a particularly large, wholly flightless, cursorial passerine should make itself known to the world. It should be a record-holder as goes size, but not necessarily be that much bigger than the largest known passerines (like lyrebirds and ravens): I’m talking about a bird that weighs 3-5 kg and is thus similar in size to a large chicken. It should be a big, long-legged rail-babbler, quail-thrush or similar, and hence be a denizen of Wallacea or nearby.
Caption: Eupetes, the Malaysian rail-babbler. A hypothetical big, flightless passerine should be a close relative of this bird. Image: Francesco Verronesi, CC BY-SA 2.0 (original here).
A few recently extinct, island-dwelling passerines were flightless, so we do know that passerines have the evolutionary potential to follow this pathway. Such species (a bunting and a few New Zealand wrens… and possibly a few others) were all small (less than 40 g).
A western Asian giant salamander. Giant salamanders (cryptobranchids) are restricted today to eastern Asia (where Andrias occurs) and North America (where Cryptobranchus occurs). Hunting, human disturbance, habitat loss and deterioration, climate change and other issues are putting them into perilous decline, right at the same time as we’re discovering that some of them are species complexes. They were more widespread in the past than they are today, since fossils show that Andrias salamanders were widespread across Europe and Asia between about 28 and 2 million years ago.
Caption: an Asian giant salamander (Andrias) photographed in captivity. Record-holding specimens of Andrias can be 1.8 m long and exceed 60 kg, and some extinct species reached even larger sizes. Image: Markus Bühler.
While there are very good reasons for the decline and extinction of the animals in the areas concerned, some of the regions where they formerly occurred still have what look like suitable habitat today and are sparsely populated by people. Furthermore, extinct giant salamanders weren’t all denizens of fast-flowing, highly oxygenated streams like those inhabited by the modern populations. Some inhabited ponds and lakes. Ergo: I would really, really like there to be a west Asian cryptobranchid that comes from a habitat considered weird for the other living members of the group. And it doesn’t have to be a giant of 2 metres or more. A hellbender-sized species of 70 cm or so will do fine thank you very much.
Caption: some extinct cryptobranchids - this is Zdeněk Burian’s reconstruction of Andrias scheuchzeri - inhabited European ponds and lakes. I’ve previously criticised this image for showing the animal as terrestrial. Since then, the proposal has been made that some extinct cryptobranchids (albeit not A. scheuchzeri) were significantly more terrestrial than living species. Image: (c) Zdeněk Burian.
Again, this is an area of special interest to cryptozoologists, since there have been occasional suggestions that stories, engravings and such from western Asia might reflect folk knowledge of unusually big salamanders in the region. In reality, the images and stories concerned are super-ambiguous and more likely refer to otters and god knows what else.
Caption: at left, an Andrias skull. Image: Darren Naish. At right: Japanese giant salamander (A. japonicus) illustration by Y. de Hoev from 1887. Image: Y. de Hoev, public domain (original here).
A living albanerpetontid. Everyone knows that there are three main groups of living amphibian: caecilians, salamanders and anurans (frogs and toads). But until (geologically) recently, there was a fourth group: the albanerpetontids, sometimes termed albies by those who work on them. Albanerpetontids were geographically widespread, their range including Eurasia, northern Africa and North America, and they were geologically long-lived too. The oldest are from the Middle Jurassic while the youngest are… well, we’ve known of Miocene fossil albanerpetontids for decades, have known of Pliocene specimens since 2005 (Venczel & Gardner 2005), and now know that at least one species persisted into the Pleistocene (Villa et al. 2018). The fact that their fossil record has been creeping towards the Recent means that the possibility of fossil and even extant Holocene specimens being discovered isn’t ridiculous, especially given the small size of these animals and hence tiny size of their bones.
Caption: new salamander species are occasionally discovered in Europe and Asia even now. It would be amazing if an animal suspected to be a ‘new salamander’ one day turned out to be a living albanerpetontid. These reconstructions were published by McGowan & Evans (1995). They might have erred in implying that the scales would be externally visible as shown here; more likely is that they were concealed by epidermis, as in other scaly fossil amphibians. Image: McGowan & Evans (1995).
To be frank, a live albanerpetontid wouldn’t be a particularly spectacular animal: it would be a tiny, slim, salamander-like amphibian less than 10 cm long, and it wouldn’t be much fun to watch since it would spend most of its time hiding and burrowing in leaf litter. But among herp-nerds it would be a huge deal. Live albanerpetontids were scaly-skinned (though the scales were not necessarily visible externally), with eyelids, and with adaptations in the snout, skull-roof, neck and body shape linked to head-first burrowing (McGowan & Evans 1995).
Caption: an artistic reconstruction of a live albanerpetontid… produced for my in-prep The Vertebrate Fossil Record. Image: Darren Naish.
A Eurasian palaeognath. Palaeognaths are the big, flightless ratites (ostriches, emus and so on), the superficially gamebird-like, flight-capable tinamous, and their extinct relatives. A huge amount has been written about the evolutionary history and biogeography of these birds, since their distribution is curious and has resulted in all kinds of different models about how they might have spread around the world. I’ve written about this issue at length on previous occasion (the articles concerned being famous for generating the longest-ever comment threads in the history of TetZoo… though all of this is mostly wasted now, what with SciAm’s paywalling of the site, sigh). Living palaeognaths are absent from Eurasia, despite the former present in the region of ancient, flight-capable Paleogene taxa, extinct ostriches and others.
Caption: as this map shows, modern palaeognaths occurred everywhere until recently (except Antarctica) with the exception of northern North America and the cooler parts of Eurasia. Extinctions across Eurasia, Madagascar and New Zealand of course saw the disappearance of various members of the group. Image: Darren Naish.
The fact that Paleogene Europe was home to many bird groups that no longer occur there but are now denizens of tropical regions elsewhere leads me to hope for a living palaeognath – a tinamou- or bustard-sized species – that descends directly from archaic Paleogene taxa and now lives in the Asian tropics. It should be a cryptic generalist with barred plumage and a mid-length bill and a reduced flight ability.
A gigantic, predatory, limbed amphisbaenian. Regular readers of TetZoo might know that I really like amphisbaenians: the mostly limbless, bullet-headed ‘worm lizards’ of the American tropics, Africa, and parts of southern Europe and western Asia. Amphisbaenian evolutionary history and biogeography has become increasingly complex in recent years as we’ve learnt a bunch of new stuff about their fossil history, genetics and anatomy. Among the weirdest of amphisbaenians are the ajolotes (or bipedids), the only extant group to possess limbs. These limbs are not small stumps or flaps (as they are in some other near-limbless, serpentine squamates) but well-developed, clawed forelimbs. According to some phylogenetic models, ajolotes are not the sister-group to limbless amphisbaenians but deeply nested within the limbless clade (Conrad 2008, Videl et al. 2008), in which case their limbedness – if you will – perhaps evolved from limbless ancestors. Add to this the fact that some amphisbaenians are robust-jawed, short-faced predators of vertebrates that ambush prey from beneath the surface and bite chunks from the bodies of surface-dwelling mammals and reptiles.
Caption: Bipes, an ajolote of Mexico (they might occur in parts of the USA as well). Three extant species are recognised. Image: Darren Naish.
So then… where oh where are the giant, limbed, robust-skulled, vertebrate-eating amphisbaenians? By ‘giant’, I am not talking about a graboid-sized monster of several metres (though that would be nice), but a more reasonable animal of a mere 1.5 metres or so. Easily the stuff of nightmares. They could inhabit warm regions of any continent.
Caption: Carl Gans’s illustration of a burrowing ajolote, showing how the large, well-clawed forelimbs function in propulsion. This is clearly a Five-toed worm lizard Bipes biporus; the other extant species have four and three digits, respectively. Image: Gans (1974).
Is there any reason to think that gigantic, predatory, limbed amphisbaenians might actually exist and await discovery? Nope. But I wish it were so. Regular readers might recognise that such creatures are denizens of the alternative-timeline Earth of the Squamozoic, but I’m sure that that’s coincidental.
Caption: what would a gigantic, predatory, limbed amphisbaenian look like? Like this, of course. Image: Darren Naish.
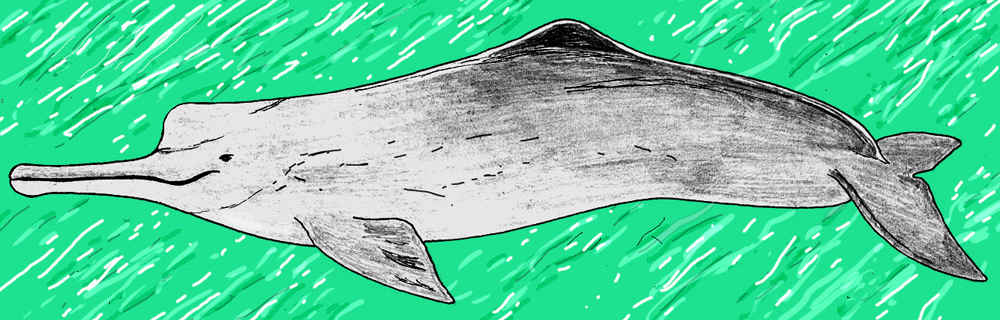
An African or west Eurasian, long-beaked river dolphin. On several occasions within the history of odontocete cetaceans (‘toothed whales’), lineages have moved into brackish and estuarine environments, and eventually made the transition to committed freshwater life. There are the Asian Platanista species, the recently extinct Lipotes of China, and the tropical American Inia species. Once united within Platanistoidea and thought to be close kin, we know today that these animals represent at least three separate transitions to the freshwater environment (the term Platanistoidea is now restricted to the Platanista lineage alone). In addition, members of other groups – I’m thinking of the delphinid Orcaella – occur in rivers within parts of their range. There’s also a fossil beaked whale that might be indicative of freshwater specialisation in yet another odontocete group (Mead 1975).
Caption: river dolphins are pretty special looking. This is a Ganges river dolphin (Platanista gangetica). Image: Zahangir Alom / Marine Mammal Commission / National Oceanic and Atmospheric Administration, public domain (original here).
In view of all this, why aren’t there river-dwelling dolphins in Africa, Europe or western Asia? Again, the answer seems to be… there just aren’t. A few fossil taxa suggest that such animals might have evolved if things had gone another way (there are fossil platanistoids from the Caucasus, for example). But I humbly submit that the great river systems of tropical Africa, the Tigris-Euphrates system of western Asia and the Danube, Po, Ebro, Dniester and others of southern Europe would be much improved if only we knew of their endemic riverine dolphins. I’m talking about a true riverine specialist, convergent with Inia and Platanista, with a long beak, spike-like teeth, reduced eyesight, the works. And if you want to play fast and loose with antiquarian literature and anecdote, there are references in the literature to ‘river dolphins’ in the Nile and there are even one or two eyewitness accounts from central Europe that describe long-beaked ‘dolphins’ seen in rivers and lakes.
Caption: if there are extant west Eurasian or African river dolphins, they should look like this. This is a hypothetical species, modelled on the American Inia and Asian Platanista. Image: Darren Naish.
An endoparasitic tetrapod. Tetrapods have become parasites on several occasions. Vampire bats are parasites of birds and mammals, and it’s even been argued that some blood- and milk-eating human populations can be considered parasites of the mammals they rely on (though the mammals concerned are domesticated, so it’s complicated). Elsewhere among vertebrates, everyone knows about the parasitic catfishes that invade the gills of other actinopterygian fishes and even the urethras of mammals; less familiar is the fact that other actinopterygians can, on rare occasion, become trapped inside the bodies of other vertebrates and then make a successful living. Yes, you read that right. I have in mind the case where two Snubnosed or Pugnose eels Simenchelys parasitica were discovered living inside the heart of a mako shark (Caira et al. 1997; see also Eagderi et al. 2016). This eel is not – despite its name* – ordinarily an internal parasite: this was a case of facultative endoparasitism!
Caption: at left, a snubnosed eel found living inside the heart of a shark. Eels are not tetrapods, it’s true. But here’s evidence that aquatic vertebrates can become endoparasites. Image: Caira et al. (1997). At right: an aquatic typhlonectid caecilian. Surely it’s only a matter of time before we discover an endoparasitic one of those as well. Image: Neil Phillips.
There are all kinds of reasons why a tetrapod couldn’t become an endoparasite, respiration being high on the list. A hypothetical endoparasitic tetrapod would have to be small, with remarkable tolerance of unusual chemical and thermal conditions, with low oxygen requirements, and most likely with the ability to respire cutaneously or via gills. In other words, it should be the world’s weirdest caecilian. As if caecilians aren’t weird enough, I’d love there to be small, endoparasitic caecilians. Given that some caecilians are already aquatic gill-breathers that will consume the tissues of fish (exhibit A: the sequence from River Monsters where Jeremy Wade discovers swarming typhlonectid caecilians in the carcass of a large fish), I predict these animals to be aquatic, South American species that parasitise actinopterygians and aquatic mammals, like Inia the river dolphin.
* Snubnosed eels were given the name ‘parasitica’ because they opportunistically latch on to the bodies of larger fish and eat away at the flesh. They were not thought to ever be proper internal parasites prior to 1992.
And that’s where I’ll stop for now. I actually came up with a list containing numerous additional ‘wish list’ animals but time is against me. Maybe I’ll cover them in another article. Whatever, this was all a bit of fun and I hope you enjoyed it.
For TetZoo articles relevant to the issues covered here, see…
Surreal caecilians part I: tentacles and protrusible eyes, January 2008
Surreal caecilians part II: pass mum’s skin, hold the mayo, January 2008
Close up to Andrias, despite the smell and the teeth, December 2010
Welcome to the Squamozoic!, April 2013
If Bigfoot Were Real, June 2016
PS I’m going to stop linking to the SciAm run of TetZoo articles soon, because I cannot access them at all and they’re now all but useless. They all need to be relocated to an open-access site.
Refs - -
Caira, J. N., Benz, G. W., Borucinska, J. & Kohler, N. E. 1997. Pugnose eels, Simenchelys parasiticus (Synaphobranchidae) from the heart of a shortfin mako, Isurus oxyrinchus (Lamnidae). Environmental Biology of Fishes 49, 139-144.
Conrad, J. 2008. Phylogeny and systematics of Squamata (Reptilia) based on morphology. Bulletin of the American Museum of Natural History 310, 1-182.
Eagderi, S., Christiaens, J., Boone, M., Jacobs, P. & Adriaens, D. 2016 Functional morphology of the feeding apparatus in Simenchelys parasitica (Simenchelyinae: Synaphobranchidae), an alleged parasitic eel. Copeia 104, 421-439.
Graham, G. L. 2002. Bats of the World. St. Martin’s Press, New York.
Mackal, R. P. 1987. A Living Dinosaur? In Search of Mokele-Mbembe. E. J. Brill, Leiden.
McGowan, G. J. & Evans, S. E. 1995. Albanerpetontid amphibians from the Cretaceous of Spain. Nature 373, 143-145.
Mead, J. G. 1975. A fossil beaked whale (Cetacea: Ziphiidae) from the Miocene of Kenya. Journal of Paleontology 49, 745-751.
Naish, D. 2017. Hunting Monsters: Cryptozoology and the Reality Behind the Myths. Arcturus, London.
Simons, E. L. & Ettel, P. C. 1970. Gigantopithecus. Scientific American 222 (1), 77-84.
Venczel, M. & Gardner, J. D. 2005. The geologically youngest albanerpetontid amphibian, from the Lower Pliocene of Hungary. Palaeontology 48, 1273-1300.
Vidal, N., Azvolinsky, A., Cruaud, C. & Hedges, S. B. 2008. Origin of tropical American burrowing reptiles by transatlantic rafting. Biology Letters 4, 115-118.
Villa, A., Blain, H.-A. & Delfino, M. 2018. The Early Pleistocene herpetofauna of Rivoli Veronese (Northern Italy) as evidence for humid and forested glacial phases in the Gelasian of Southern Alps. Palaeogeography, Palaeoclimatology, Palaeoecology 490, 393-403.
Whatever Happened to the Kabomani Tapir?
As a regular TetZoo reader, you’ll no doubt be aware of Tapirus kabomani, the (alleged) new South American tapir species named by Mario Cozzuol and colleagues in 2013 (Cozzuol et al. 2013).
Caption: Tapirus… poster-child for TetZoo Park. Image: Patrick Murphy.
T. kobamani – popularly termed simply the Kabomani tapir (the names Little black tapir or Black dwarf tapir are also available; read on) – was described as smaller and darker than other South American tapirs, and as possessing a few distinctive osteological features, like a broad forehead and frontal bones that are more inflated than those of other tapirs. Its validity as a distinct species is, however, controversial, as we’ll see.
Caption: there’s TetZoo HAVE YOU HEARD ABOUT THE NEW TAPIR merchandise. Go here.
I wrote about the initial naming and description of T. kabomani back here at TetZoo ver 3 (warning: now paywalled). Immediately there was a reasonable amount of scepticism from other zoologists familiar with tapirs and their biology, distribution and systematics – many of whom argued that T. kabomani was similar enough to the Brazilian or Lowland tapir T. terrestris to be considered conspecific with it – and also a claim that exactly the same sort of tapir had already been described by another zoologist (namely, Marc van Roosmalen).
Caption: one of several T. kabomani images captured by remote cameras: from Cozzuol et al. (2013). Image: Cozzuol et al. (2013).
But what has happened since 2013? Well, quite a bit: several studies evaluating the status of T. kabomani have been published since Cozzuol et al.’s initial paper of 2013. What do these various studies state, and what do they conclude? Let’s look at each of the studies in turn. I’ve done my best to summarise the relevant papers, and to keep my summaries brief.
As noted above, Marc van Roosmalen has been stating right from the time that kabomani was first described that it is identical with another alleged small tapir – T. pygmaeus, termed the Black dwarf tapir by van Roosmalen – that he named (online) in 2002, briefly diagnosed and described in popular books of 2008 and 2013, and formally published within a section of another book of 2013 (Van Roosmalen & Van Hooft 2013). Van Roosmalen (2014) petitioned the ICZN (case 3650) to have the 2013 book in question – Barefoot Through the Amazon – On the Path of Evolution – registered as part of the ‘Official List of Works Approved as Available for Zoological Nomenclature’, and thus to have T. pygmaeus endorsed as a valid, official name with priority over T. kabomani. A ruling on this proposal has not been made, to my knowledge. Van Roosmalen again stated his view that these two forms are synonymous in a 2015 publication (Van Roosmalen 2015).
Caption: the Black dwarf tapir, as illustrated in Marc van Roosmalen’s 2013 book. Image: van Roosmalen (2013).
Robert Voss et al. (2014) were highly critical of Cozzuol et al.’s (2013) case for the validity of T. kabomani, literally starting their paper with reference to Carl Sagan’s aphorism that “extraordinary claims require extraordinary evidence” (p. 893). To be honest, I’m not sure that the reporting of a new species of tapir is as extraordinary as they think it is but… whatever, they argued that none of the molecular, morphological or ethnological evidence compiled by Cozzuol et al. (2013) withstood scrutiny. Their most interesting contention was that kabomani is not ‘distinct enough’ from T. terrestris to warrant species-level separation (the sequence divergence in Cytb amounting to 1.3%), and that both T. kabomani and the Mountain or Woolly tapir T. pinchaque are not phylogenetically separate from T. terrestris, both failing to be recovered as reciprocally monophyletic (Voss et al. 2014). Remember that this affects T. pinchaque as well as T. kabomani: we’ll be coming back to that point.
Caption: this section of Voss et al.’s (2014) molecular phylogeny shows T. pinchaque and T. kabomani as poorly differentiated from T. terrestris. Image: Voss et al. (2014).
Mario Cozzuol et al. (2014) published a response to Voss et al. (2014). With regard to Voss et al.’s (2014) contention (“Have several generations of Neotropical mammalogists really failed to recognise a species of Recent megafauna that is said to be widely distributed in Amazonia?”), their specific response was: “The answer is, simply, yes. Specifically, they failed, as many others have done for many years, to listen to the local people more carefully; those people have been aware of the existence of this species for a long time” (p. 899). Cozzuol et al. (2014) re-iterated their view that kabomani is morphologically distinct (they noted in particular its sagittal crest), and that it is recognised as distinct by various Amazonian peoples. Regarding Voss et al.’s (2014) finding that kabomani is nested within T. terrestris, Cozzuol et al. (2014) reported that different molecular trees were recovered depending on which tapir taxon was used as the outgroup, and that the nesting of kabomani within T. terrestris did not disprove its status as a distinct species given that T. pinchaque (the Mountain tapir, universally regarded as a distinct species) was found to be intractable from the terrestris-kabomani clade based on genetic data (Cozzuol et al. 2014).
Caption: portrait of T. kabomani, produced by G. Braga to accompany Cozzuol et al.’s (2013) original paper. Image: Cozzuol et al. (2013).
Manuel Ruiz-García et al. (2015) examined mitochondrial gene diversity across all South American tapirs, their aim being to better understand the genetic history of the group and thus its evolution, systematics and conservation biology. They included data from five kabomani specimens: two were the Brazilian specimens analysed by Cozzuol et al. (2013), and the others were individuals from Colombia, Peru and Ecuador (Ruiz-García et al. 2015). They found kabomani tapirs to be “more closely related to T. terrestris than to the other tapir species”, reported “significant differences with [DN – I think they meant ‘from’] T. terrestris as well as with [DN – surely ‘from’] T. pinchaque” (p. 11), but overall found kabomani tapirs to “yield lower genetic distances with regard to T. terrestris than did T. pinchaque” (p. 14). In other words, kabomani tapirs were not – in their view – genetically ‘distinct enough’ to warrant species status and should more reasonably be considered a distinct population of T. terrestris. This view was modified later in the text where they specifically stated (and depicted) kabomani tapirs as a clade within T. terrestris, the divergence of the kabomani lineage being suggested to have occurred between 1.3 million and 360,000 years ago (p. 18). Such a divergence date is young relative to other divergences between extant tapir species (some of you might recall date of divergence being deemed relevant in the debate surrounding white rhino phylogeny and taxonomy). These authors also provided a lengthy critique of anatomical criteria used by Cozzuol et al. (2013) to differentiate T. kabomani, arguing that some ‘kabomani-type’ tapirs did not have the morphological features supposedly diagnostic for this taxon, and furthermore than some small, ‘kabomani-type’ animals are not of the genetic kabomani group. Bottomline: kabomani “is a particular lineage within T. terrestris” (p. 34), and it is not morphologically well differentiated from the rest of T. terrestris, some other populations of which look kabomani-like.
Caption: Ruiz-García et al.’s (2015) maximum likelihood tree, incoporated mitochondrial gene data for 93 tapir specimens. T. pinchaque is blue, T. terrestris is red, T. kabomani is green, and T. bairdii is purple. Note that kabomani is nested within T. terrestris. Image: Ruiz-García et al.’s (2015).
Dumbá et al. (2018) analysed skull shape variation in living tapir species (though they also included a few fossil ones too), and specifically analysed kabomani. They found kabomani skulls to have some overlap in morphospace with T. terrestris though noted that both could still be distinguished, in part because of kabomani’s broad forehead. In some landmark-based analyses, the kabomani sample overlapped almost entirely with the region of morphospace occupied by T. terrestris. The assumption of this study appears to be that kabomani is distinct and only similar to T. terrestris when certain sets of cranial landmarks are used (note that Mario Cozzual is the study’s last author). Another interpretation could be that kabomani overlaps so extensively with T. terrestris that it should be regarded as a poorly differentiated ‘extension’ of the morphospace occupied by T. terrestris.
Caption: the landmark-based morphometric work published recently by Dumbá et al. (2018) shows T. kabomani to overlap quite extensively with the morphospace occupied by T. terrestris. Image: Dumbá et al. (2018).
What, then, to conclude? By now you’ve surely heard, on a great many occasions, the contention that the entities we call ‘species’ do not have a consistent, well defined definition across all tetrapods, let alone across all animals or all organisms. Whether a given population warrants recognition as a ‘species’ is still, to some considerable degree, a subjective issue. Having gotten that caveat out of the way, most (note: most) mammalogists would agree that the minor molecular and morphological differences separating the Kabomani tapir from T. terrestris are not compelling, and it does appear most likely that it is a variant, morph or lineage of T. terrestris. However…
Caption: the Mountain or Woolly tapir was posited by Cozzuol et al. (2013) as closer to T. terrestris than is T. kabomani, but the inverse was recovered by Ruiz-García et al. (2015). Image: Just Chaos, CC BY-SA 2.0 (original here).
Problem 1: Voss et al.’s (2014) initial claim that the Mountain tapir is as much as part of T. terrestris as is kabomani was always problematic. After all, everyone agrees that the Mountain tapir should be retained as a valid species; if the Mountain tapir were to be regarded as part of T. terrestris, people would likely use special pleading to keep it distinct… which would, in turn, then negate the claim that kabomani was not worthy of species status. However, the more comprehensive analysis compiled by Ruiz-García et al. (2015) seems to have resolved this issue. Problem 2: the implication that the recovery of kabomani as a lineage within T. terrestris automatically negates its status as a species is not entirely fair or technically correct, since there are a great many animal populations we term ‘species’ that are not monophyletic and/or not outside other populations also termed ‘species’. A classic example is the Polar bear Ursus maritimus (generally found in studies to be nested within the Brown bear U. arctos.) but there are many, many others. In other words, finding kabomani to be a lineage within T. terrestris does not automatically negate a species-level status. But is it ‘distinct enough’ to be regarded as a ‘species’ all its own? My conclusion from all the work discussed above: no, no it is not, alas.
Caption: Tapirus terrestris is a pretty variable animal, seemingly with a complex evolutionary history and a degree of morphological variation that’s only now beginning to come to light. This captive individual is from Chester Zoo, UK. Image: Darren Naish.
And that is where we end for now. Tapirs have been covered quite a few times on TetZoo now, though once again I will note that many of the articles concerned are now paywalled due to a recent decision made at Scientific American blogs. If you can access them, the articles are here…
Multiple new species of large, living mammal (part I), June 2007
Because you can never have too many tapirs, September 2009
The biggest tapir, September 2009
Tapir attacks past, present, but hopefully not future, August 2013
A new living species of large mammal: hello, Tapirus kabomani!, December 2013
World Tapir Day, 2015, April 2015
On World Tapir Day, a Quick Look at (Part of) Tapir History, April 2016
Refs - -
Cozzuol , M. A., Clozato, C. L. , Holanda, E. C., Rodrigues, F. H. G., Nienow, S., de Thoisy, B., Redondo, R. A. F. & Santos, F. R. 2013. A new species of tapir from the Amazon. Journal of Mammalogy 94, 1331-1345.
Cozzuol, M. A., de Thoisy, B., Fernandes-Ferreira, H., Rodrigues, F. H. G. & Santos, F. R. 2014. How much evidence is enough evidence for a new species? Journal of Mammalogy 95, 899-905.
Dumbá, L. C. C. S., Parisi Dutra, R. & Cozzuol, M. A. 2018. Cranial geometric morphometric analysis of the genus Tapirus (Mammalia, Perissodactyla). Journal of Mammalian Evolution https://doi.org/10.1007/s10914-018-9432-2
Ruiz-García, M., Castellanos, A., Agueda Bernal, L., Navas, D., Pinedo-Castro, M. & Mark Shostell, J. 2015. Mitochondrial gene diversity of the mega-herbivorous species of the genus Tapirus (Tapiridae, Perissodactyla) in South America and some insights on their genetic conservation, systematics and the Pleistocene influence on their genetic characteristics. Advances in Genetic Research 14, 1-51.
Van Roosmalen, M. G. M. 2014. Case 3650: Tapirus pygmaeus Van Roosmalen & Van Hooft in Van Roosmalen, 2013 (Mammalia, Perissodactyla, TAPIRIDAE): proposed confirmation of availability of the specific name and of the book in which this nominal species was proposed. Bulletin of Zoological Nomenclature 71, 84-87.
Van Roosmalen, M. G. M. 2015. Hotspot of new megafauna found in the Central Amazon (Brazil): the lower Rio Aripuanã Basin. Biodiversity Journal 6, 219-244.
Van Roosmalen, M. G. M. & Van Hooft, P. 2013. New species of living tapir, the dwarf tapir (Mammalia: Tapiridae) from the Brazilian Amazon, in Van Roosmalen, M. G. M. (ed), Barefoot Through the Amazon – On the Path of Evolution. CreateSpace, North Charleston SC, pp. 400-404.
Voss, R. S., Helgen, K. M. & Jansa, S. A. 2014. Extraordinary claims require extraordinary evidence: a comment on Cozzuol et al. (2013). Journal of Mammalogy 95, 893-898.